Related Research Articles

Biopolymers are polymers produced by living organisms; in other words, they are polymeric biomolecules. Biopolymers contain monomeric units that are covalently bonded to form larger structures. There are three main classes of biopolymers, classified according to the monomeric units used and the structure of the biopolymer formed: polynucleotides, which are long polymers composed of 13 or more nucleotide monomers; polypeptides, which are short polymers of amino acids; and polysaccharides, which are often linear bonded polymeric carbohydrate structures. Other examples of biopolymers include rubber, suberin, melanin and lignin.

A carbohydrate is a biomolecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen–oxygen atom ratio of 2:1 (as in water) and thus with the empirical formula Cm(H2O)n (where m may be different from n). This formula holds true for monosaccharides. Some exceptions exist; for example, deoxyribose, a sugar component of DNA, has the empirical formula C5H10O4. The carbohydrates are technically hydrates of carbon; structurally it is more accurate to view them as aldoses and ketoses.
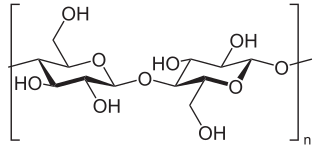
Cellulose is an organic compound with the formula (C
6H
10O
5)
n, a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall of green plants, many forms of algae and the oomycetes. Some species of bacteria secrete it to form biofilms. Cellulose is the most abundant organic polymer on Earth. The cellulose content of cotton fiber is 90%, that of wood is 40–50%, and that of dried hemp is approximately 57%.
A monomer is a molecule that "can undergo polymerization thereby contributing constitutional units to the essential structure of a macromolecule". Large numbers of monomers combine to form polymers in a process called polymerization.

Polysaccharides are polymeric carbohydrate molecules composed of long chains of monosaccharide units bound together by glycosidic linkages, and on hydrolysis give the constituent monosaccharides or oligosaccharides. They range in structure from linear to highly branched. Examples include storage polysaccharides such as starch and glycogen, and structural polysaccharides such as cellulose and chitin.

Starch or amylum is a polymeric carbohydrate consisting of a large number of glucose units joined by glycosidic bonds. This polysaccharide is produced by most green plants as energy storage. It is the most common carbohydrate in human diets and is contained in large amounts in staple foods like potatoes, wheat, maize (corn), rice, and cassava.

Dextrins are a group of low-molecular-weight carbohydrates produced by the hydrolysis of starch or glycogen. Dextrins are mixtures of polymers of D-glucose units linked by α-(1→4) or α-(1→6) glycosidic bonds.
Polymer chemistry is a sub-discipline of chemistry that focuses on the chemical synthesis, structure, chemical and physical properties of polymers and macromolecules. The principles and methods used within polymer chemistry are also applicable through a wide range of other chemistry sub-disciplines like organic chemistry, analytical chemistry, and physical chemistry Many materials have polymeric structures, from fully inorganic metals and ceramics to DNA and other biological molecules, however, polymer chemistry is typically referred to in the context of synthetic, organic compositions. Synthetic polymers are ubiquitous in commercial materials and products in everyday use, commonly referred to as plastics, and rubbers, and are major components of composite materials. Polymer chemistry can also be included in the broader fields of polymer science or even nanotechnology, both of which can be described as encompassing polymer physics and polymer engineering.
An excipient is a substance formulated alongside the active ingredient of a medication, included for the purpose of long-term stabilization, bulking up solid formulations that contain potent active ingredients in small amounts, or to confer a therapeutic enhancement on the active ingredient in the final dosage form, such as facilitating drug absorption, reducing viscosity, or enhancing solubility. Excipients can also be useful in the manufacturing process, to aid in the handling of the active substance concerned such as by facilitating powder flowability or non-stick properties, in addition to aiding in vitro stability such as prevention of denaturation or aggregation over the expected shelf life. The selection of appropriate excipients also depends upon the route of administration and the dosage form, as well as the active ingredient and other factors. A comprehensive classification system based on structure-property-application relationships has been proposed for excipients used in parenteral medications.
A binder or binding agent is any material or substance that holds or draws other materials together to form a cohesive whole mechanically, chemically, by adhesion or cohesion.

A thickening agent or thickener is a substance which can increase the viscosity of a liquid without substantially changing its other properties. Edible thickeners are commonly used to thicken sauces, soups, and puddings without altering their taste; thickeners are also used in paints, inks, explosives, and cosmetics.

Bioplastics are plastics derived from renewable biomass sources, such as vegetable fats and oils, corn starch, straw, woodchips, food waste, etc. Bioplastic can be made from agricultural by-products and also from used plastic bottles and other containers using microorganisms. Common plastics, such as fossil-fuel plastics are derived from petroleum or natural gas. Not all bioplastics are biodegradable nor biodegrade more readily than commodity fossil-fuel derived plastics. Bioplastics are usually derived from sugar derivatives, including starch, cellulose, and lactic acid. As of 2014, bioplastics represented approximately 0.2% of the global polymer market.
A glucan is a polysaccharide derived from D-glucose, linked by glycosidic bonds. Beta-glucans, such as laminarin, are important energy reserve polysaccharides in macroalgae, constituting up to 35% of the dried weight of the macroalgal biomass in several brown seaweed. Many beta-glucans are medically important, showing antiinflammatory, immunostimulatory, antioxidant, anticoagulant, antiviral, antiproliferative, antiapoptosis and antitumour properties. They represent a drug target for antifungal medications of the echinocandin class.
Cellodextrins are glucose polymers of varying length resulting from cellulolysis, the breakdown of cellulose.
Cellulose acetate phthalate (CAP), also known as cellacefate (INN) and cellulosi acetas phthalas, is a commonly used polymer phthalate in the formulation of pharmaceuticals, such as the enteric coating of tablets or capsules and for controlled release formulations. It is a cellulose polymer where about half of the hydroxyls are esterified with acetyls, a quarter are esterified with one or two carboxyls of a phthalic acid, and the remainder are unchanged. It is a hygroscopic white to off-white free-flowing powder, granules, or flakes. It is tasteless and odorless, though may have a weak odor of acetic acid. Its main use in pharmaceutics is with enteric formulations. It can be used together with other coating agents, e.g. ethyl cellulose. Cellulose acetate phthalate is commonly plasticized with diethyl phthalate, a hydrophobic compound, or triethyl citrate, a hydrophilic compound; other compatible plasticizers are various phthalates, triacetin, dibutyl tartrate, glycerol, propylene glycol, tripropionin, triacetin citrate, acetylated monoglycerides, etc.
Association theory is a discredited theory first advanced by chemist Thomas Graham in 1861 to describe the molecular structure of substances such as cellulose and starch, now understood to be polymers. Association theory postulates that such materials are composed of a collection of smaller molecules bound together by an unknown force. Graham termed these materials colloids. Prior to the development of macromolecular theory by Hermann Staudinger in the 1920s, association theory remained the most prevalent model of polymer structure in the scientific community.
Cellulose fibers are fibers made with ethers or esters of cellulose, which can be obtained from the bark, wood or leaves of plants, or from other plant-based material. In addition to cellulose, the fibers may also contain hemicellulose and lignin, with different percentages of these components altering the mechanical properties of the fibers.
The molecular formula C6H10O5, of molecular weight 162.14, may refer to:

Paper chemicals designate a group of chemicals that are used for paper manufacturing, or modify the properties of paper. These chemicals can be used to alter the paper in many ways, including changing its color and brightness, or by increasing its strength and resistance to water.
The surface chemistry of paper is responsible for many important paper properties, such as gloss, waterproofing, and printability. Many components are used in the paper-making process that affect the surface.
References
- ↑ Thomsen, Lars. "International Starch: Starch and Glucose Glossary". www.starch.dk. Retrieved 2017-05-30.
- ↑ Varshney, V. K.; Naithani, Sanjay (2011). Kalia, Susheel; Kaith, B. S.; Kaur, Inderjeet, eds. Cellulose Fibers: Bio- and Nano-Polymer Composites. Springer Berlin Heidelberg. pp. 43–60. doi:10.1007/978-3-642-17370-7_2. ISBN 9783642173691.