Related Research Articles
Antioxidants are compounds that inhibit oxidation, a chemical reaction that can produce free radicals. Autoxidation leads to degradation of organic compounds, including living matter. Antioxidants are frequently added to industrial products, such as polymers, fuels, and lubricants, to extend their usable lifetimes. Foods are also treated with antioxidants to forestall spoilage, in particular the rancidification of oils and fats. In cells, antioxidants such as glutathione, mycothiol or bacillithiol, and enzyme systems like superoxide dismutase, can prevent damage from oxidative stress.

Catalysis is the increase in rate of a chemical reaction due to an added substance known as a catalyst. Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quickly, very small amounts of catalyst often suffice; mixing, surface area, and temperature are important factors in reaction rate. Catalysts generally react with one or more reactants to form intermediates that subsequently give the final reaction product, in the process of regenerating the catalyst.

Superoxide dismutase (SOD, EC 1.15.1.1) is an enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide (O−
2) radical into ordinary molecular oxygen (O2) and hydrogen peroxide (H
2O
2). Superoxide is produced as a by-product of oxygen metabolism and, if not regulated, causes many types of cell damage. Hydrogen peroxide is also damaging and is degraded by other enzymes such as catalase. Thus, SOD is an important antioxidant defense in nearly all living cells exposed to oxygen. One exception is Lactobacillus plantarum and related lactobacilli, which use a different mechanism to prevent damage from reactive O−
2.

Metalloprotein is a generic term for a protein that contains a metal ion cofactor. A large proportion of all proteins are part of this category. For instance, at least 1000 human proteins contain zinc-binding protein domains although there may be up to 3000 human zinc metalloproteins.

Obligate anaerobes are microorganisms killed by normal atmospheric concentrations of oxygen (20.95% O2). Oxygen tolerance varies between species, with some species capable of surviving in up to 8% oxygen, while others lose viability in environments with an oxygen concentration greater than 0.5%.

Biohydrogen is H2 that is produced biologically. Interest is high in this technology because H2 is a clean fuel and can be readily produced from certain kinds of biomass, including biological waste. Furthermore some photosynthetic microorganisms are capable to produce H2 directly from water splitting using light as energy source.
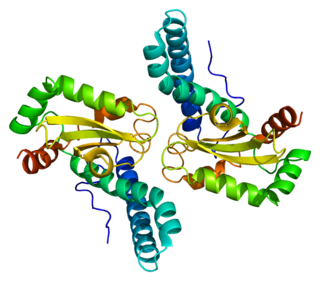
Superoxide dismutase 2, mitochondrial (SOD2), also known as manganese-dependent superoxide dismutase (MnSOD), is an enzyme which in humans is encoded by the SOD2 gene on chromosome 6. A related pseudogene has been identified on chromosome 1. Alternative splicing of this gene results in multiple transcript variants. This gene is a member of the iron/manganese superoxide dismutase family. It encodes a mitochondrial protein that forms a homotetramer and binds one manganese ion per subunit. This protein binds to the superoxide byproducts of oxidative phosphorylation and converts them to hydrogen peroxide and diatomic oxygen. Mutations in this gene have been associated with idiopathic cardiomyopathy (IDC), premature aging, sporadic motor neuron disease, and cancer.

Irwin Fridovich was an American biochemist who, together with his graduate student Joe M. McCord, discovered the enzymatic activity of copper-zinc superoxide dismutase (SOD),—to protect organisms from the toxic effects of superoxide free radicals formed as a byproduct of normal oxygen metabolism. Subsequently, Fridovich's research group also discovered the manganese-containing and the iron-containing SODs from Escherichia coli and the mitochondrial MnSOD (SOD2), now known to be an essential protein in mammals. He spent the rest of his career studying the biochemical mechanisms of SOD and of biological superoxide toxicity, using bacteria as model systems. Fridovich was also Professor Emeritus of Biochemistry at Duke University.
Dioxygen plays an important role in the energy metabolism of living organisms. Free oxygen is produced in the biosphere through photolysis of water during photosynthesis in cyanobacteria, green algae, and plants. During oxidative phosphorylation in cellular respiration, oxygen is reduced to water, thus closing the biological water-oxygen redox cycle.

Manganese is an essential biological element in all organisms. It is used in many enzymes and proteins. It is essential in plants.

JoAnne Stubbe is an American chemist best known for her work on ribonucleotide reductases, for which she was awarded the National Medal of Science in 2009. In 2017, she retired as a Professor of Chemistry and Biology at the Massachusetts Institute of Technology.
Joan Selverstone Valentine is a biological inorganic chemist and biochemist. Valentine's current work examines the role of transition metals, metalloenzymes, and oxidative stress in health. Her foremost expertise is superoxide anion and its functional enzyme superoxide dismutase. Valentine has been a member of the faculty of the University of California, Los Angeles since 1980. She served as Associate Editor of the journal Inorganic Chemistry from 1989 to 1995, and has served as Editor-in-Chief of Accounts of Chemical Research since 1994. In 2005, she was elected to the National Academy of Sciences.

Vincent L. Pecoraro, professor at the University of Michigan, is a researcher in bioinorganic chemistry and inorganic chemistry. He is a specialist in the chemistry and biochemistry of manganese, vanadium, and metallacrown chemistry. He is a fellow of the American Association for the Advancement of Science
Non-Homologous Isofunctional Enzymes (NISE) are two evolutionarily unrelated enzymes that catalyze the same chemical reaction. Enzymes that catalyze the same reaction are sometimes referred to as analogous as opposed to homologous, however it is more appropriate to name them as Non-homologous Isofunctional Enzymes, hence the acronym (NISE). These enzymes all serve the same end function but do so in different organisms without detectable similarity in primary and possibly tertiary structures.
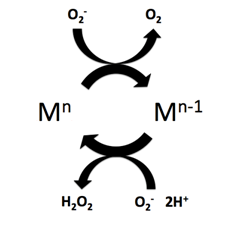
Superoxide dismutase (SOD) mimetics are synthetic compounds that mimic the native superoxide dismutase enzyme. SOD mimetics effectively convert the superoxide anion, a reactive oxygen species, into hydrogen peroxide, which is further converted into water by catalase. Reactive oxygen species are natural byproducts of cellular respiration and cause oxidative stress and cell damage, which has been linked to causing cancers, neurodegeneration, age-related declines in health, and inflammatory diseases. SOD mimetics are a prime interest in therapeutic treatment of oxidative stress because of their smaller size, longer half-life, and similarity in function to the native enzyme.
Evolution of metal ions in biological systems refers to the incorporation of metallic ions into living organisms and how it has changed over time. Metal ions have been associated with biological systems for billions of years, but only in the last century have scientists began to truly appreciate the scale of their influence. Major and minor metal ions have become aligned with living organisms through the interplay of biogeochemical weathering and metabolic pathways involving the products of that weathering. The associated complexes have evolved over time.
The Local Section of the American Chemical Society has awarded the Herty Medal since 1933 in honor of Charles Herty. The medallion is solid gold and is inscribed with the words "pro scientia et patria - Herty 1933." The Latin phrase translates roughly as "for science and country".
Julia A. Kovacs is an American chemist specializing in bioinorganic chemistry. She is professor of chemistry at the University of Washington. Her research involves synthesizing small-molecule mimics of the active sites of metalloproteins, in order to investigate how cysteinates influence the function of non-heme iron enzymes, and the mechanism of the oxygen-evolving complex (OEC).
R. David Britt is the Winston Ko Chair and Distinguished Professor of Chemistry at the University of California, Davis. Britt uses electron paramagnetic resonance (EPR) spectroscopy to study metalloenzymes and enzymes containing organic radicals in their active sites. Britt is the recipient of multiple awards for his research, including the Bioinorganic Chemistry Award in 2019 and the Bruker Prize in 2015 from the Royal Society of Chemistry. He has received a Gold Medal from the International EPR Society (2014), and the Zavoisky Award from the Kazan Scientific Center of the Russian Academy of Sciences (2018). He is a Fellow of the American Association for the Advancement of Science and of the Royal Society of Chemistry.
Connie C. Lu is a Taiwanese-American inorganic chemist and a professor of chemistry at the University of Bonn. She was previously a professor of chemistry at the University of Minnesota, Twin Cities. Lu's research focuses on the synthesis of novel bimetallic coordination complexes, as well as metal-organic frameworks. These molecules and materials are investigated for the catalytic conversion of small molecules like as N2 and CO2 into value-added chemicals like ammonia and methanol. Lu is the recipient of multiple awards for her research, including the National Science Foundation CAREER Award and the Sloan Research Fellowship in 2013, and an Early Career Award from the University of Minnesota's Initiative for Renewable Energy and the Environment in 2010.
References
- 1 2 "This year's Wingard Medalist". 1982.
- ↑ "A Consummate Student" (PDF). 1982.
- ↑ Miller, Anne-Frances (1989). Assembly of the catalytic manganese complex of photosystem II (Thesis). OCLC 23972966.[ page needed ]
- ↑ "MILLER, Anne-Frances". www.asbmb.org. Retrieved 2023-11-04.
- 1 2 3 "Anne-Frances Miller to deliver 2023 A&S Distinguished Professor Lecture | University of Kentucky College of Arts & Sciences". chem.as.uky.edu. Retrieved 2023-11-04.
- 1 2 "UK Chemist Wins 2019 Lyons Award". UKNow. 2019-05-02. Retrieved 2023-11-04.
- ↑ "Michael and Kate Barany Award for Young Investigators". University of Kentucky. Retrieved 2023-11-04.
- ↑ "Herty Medalists (1933-2023)". ACS Georgia Section. 2019-11-12. Retrieved 2023-11-04.
- ↑ "Anne-Frances Miller – Einstein Foundation Berlin". www.einsteinfoundation.de. Retrieved 2023-11-04.
- ↑ "Anne-Frances Miller named 2022-23 College of Arts and Sciences Distinguished Professor". UKNow. 2022-09-13. Retrieved 2023-11-04.
- ↑ "Electron Bifurcation - 2023 Faraday Horizon Prize winner". Royal Society of Chemistry. Retrieved 2023-11-04.