Related Research Articles

Antidepressants are a class of medications used to treat major depressive disorder, anxiety disorders, chronic pain, and addiction.
An anxiolytic is a medication or other intervention that reduces anxiety. This effect is in contrast to anxiogenic agents which increase anxiety. Anxiolytic medications are used for the treatment of anxiety disorders and their related psychological and physical symptoms.

Paroxetine, sold under the brand name Paxil among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used to treat major depressive disorder, obsessive-compulsive disorder, panic disorder, social anxiety disorder, post-traumatic stress disorder, generalized anxiety disorder, and premenstrual dysphoric disorder. It has also been used in the treatment of premature ejaculation and hot flashes due to menopause. It is taken orally.

Sertraline, sold under the brand name Zoloft among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. The effectiveness of sertraline for depression is similar to that of other antidepressants, and the differences are mostly confined to side effects. Sertraline is better tolerated than the older tricyclic antidepressants. Sertraline is effective for panic disorder, social anxiety disorder, generalized anxiety disorder (GAD), and obsessive–compulsive disorder (OCD). Although approved for post-traumatic stress disorder (PTSD), sertraline leads to only modest improvement in this condition. Sertraline also alleviates the symptoms of premenstrual dysphoric disorder (PMDD) and can be used in sub-therapeutic doses or intermittently for its treatment.
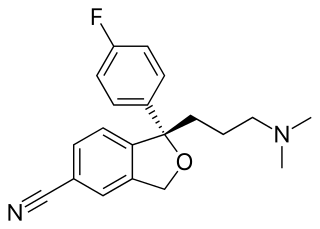
Escitalopram, sold under the brand names Lexapro and Cipralex, among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. Escitalopram is mainly used to treat major depressive disorder and generalized anxiety disorder. It is taken by mouth, available commercially as an oxalate salt exclusively.

Citalopram, sold under the brand name Celexa among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is used to treat major depressive disorder, obsessive compulsive disorder, panic disorder, and social phobia. The antidepressant effects may take one to four weeks to occur. It is typically taken orally. In some European countries, it is sometimes given intravenously to initiate treatment, before switching to the oral route of administration for continuation of treatment. It has also been used intravenously in other parts of the world in some other circumstances.

Fluvoxamine, sold under the brand name Luvox among others, is an antidepressant of the selective serotonin reuptake inhibitor (SSRI) class. It is primarily used to treat major depressive disorder and obsessive–compulsive disorder (OCD), but is also used to treat anxiety disorders such as panic disorder, social anxiety disorder, and post-traumatic stress disorder.

Mirtazapine, sold under the brand name Remeron among others, is an atypical tetracyclic antidepressant, and as such is used primarily to treat depression. Its effects may take up to four weeks but can also manifest as early as one to two weeks. It is often used in cases of depression complicated by anxiety or insomnia. The effectiveness of mirtazapine is comparable to other commonly prescribed antidepressants. It is taken by mouth.

Clomipramine, sold under the brand name Anafranil among others, is a tricyclic antidepressant (TCA). It is used in the treatment of various conditions, most notably obsessive–compulsive disorder but also many other disorders, including hyperacusis, panic disorder, major depressive disorder, trichotillomania, body dysmorphic disorder and chronic pain. It has also been notably used to treat premature ejaculation and the cataplexy associated with narcolepsy.
Forest Laboratories was a company in the pharmaceutical industry incorporated in Delaware, with its principal office in New York City. It was known for licensing European pharmaceuticals for sale in the United States. On July 1, 2014, the company was acquired by Actavis.
The second-generation antidepressants are a class of antidepressants characterized primarily by the era of their introduction, approximately coinciding with the 1970s and 1980s, rather than by their chemical structure or by their pharmacological effect. As a consequence, there is some controversy over which treatments actually belong in this class.
Allosteric serotonin reuptake inhibitor is a type of selective serotonin reuptake inhibitor (SSRI).

A serotonin reuptake inhibitor (SRI) is a type of drug which acts as a reuptake inhibitor of the neurotransmitter serotonin by blocking the action of the serotonin transporter (SERT). This in turn leads to increased extracellular concentrations of serotonin and, therefore, an increase in serotonergic neurotransmission. It is a type of monoamine reuptake inhibitor (MRI); other types of MRIs include dopamine reuptake inhibitors and norepinephrine reuptake inhibitors.

Vortioxetine, sold under the brand name Trintellix and Brintellix among others, is an antidepressant of the serotonin modulator and stimulator (SMS) class. Its effectiveness is viewed as similar to that of other antidepressants. It is taken orally.

Selective serotonin reuptake inhibitors (SSRIs) are a class of drugs that are typically used as antidepressants in the treatment of major depressive disorder, anxiety disorders, and other psychological conditions.

Tedatioxetine is an experimental antidepressant that was discovered by scientists at Lundbeck; in 2007 Lundbeck and Takeda entered into a partnership that included tedatioxetine but was focused on another, more advanced Lundbeck drug candidate, vortioxetine.
Intractable pain, also called intractable pain syndrome (IPS), is a severe, constant, relentless, and debilitating pain that is not curable by any known means and which causes a house-bound or bed-bound state and early death if not adequately treated, usually with opioids and/or interventional procedures. It is not relieved by ordinary medical, surgical, nursing, or pharmaceutical measures. Unlike the more common chronic pain, it causes adverse biologic effects on the body's cardiovascular, hormone, and neurologic systems. Patients experience changes in testosterone, estrogen, cortisol, thyroid hormones, and/or pituitary hormones. Both men and women require testosterone, however many doctors neglect to test women for low testosterone. Untreated intractable pain can cause death.
Selective serotonin reuptake inhibitors, or serotonin-specific re-uptake inhibitor (SSRIs), are a class of chemical compounds that have application as antidepressants and in the treatment of depression and other psychiatric disorders. SSRIs are therapeutically useful in the treatment of panic disorder (PD), posttraumatic stress disorder (PTSD), social anxiety disorder, obsessive-compulsive disorder (OCD), premenstrual dysphoric disorder (PMDD), and anorexia. There is also clinical evidence of the value of SSRIs in the treatment of the symptoms of schizophrenia and their ability to prevent cardiovascular diseases.
References
- ↑ Kanba S (Jan 1999). "Disparities in drug development: the Japanese paradox". J Psychiatry Neurosci. 24 (1): 13–4. PMC 1188972 . PMID 9987203. Archived from the original on 2016-03-06. Retrieved 2007-09-07.
- 1 2 3 Berger D (Sep 2005). "Antidepressant clinical development in Japan: Current perspectives and future horizons" (PDF). Clinical Research Focus. 16 (7): 32–5.
- ↑ Berger D, Fukunishi I (Jul 1996). "Psychiatric drug development in Japan". Science. 273 (5273): 318–9. Bibcode:1996Sci...273..318B. doi:10.1126/science.273.5273.318. PMID 8685717. S2CID 20878837.
- ↑ Kamimura M, Aoba A (2002). "Drug Therapy for Depression in Japan" (PDF).
- ↑ "Search results detail| Kusurino-Shiori(Drug information Sheet)". www.rad-ar.or.jp. Retrieved 2020-03-16.
- ↑ Asakura S, Koyama T, Hosokai T, Kawano H, Kajii Y (2014-10-30). "Post-Marketing Surveillance of Fluvoxamine Maleate Used Long-Term in Patients with Social Anxiety Disorder in Japan". Drugs - Real World Outcomes. 1 (1): 7–19. doi:10.1007/s40801-014-0005-2. ISSN 2199-1154. PMC 4883186 . PMID 27747476.
- ↑ Life Sciences World - Online resource for biotechnology, pharmaceutical, medical devices and life sciences industries.
- ↑ "FY2006 List of Approved Products: New Drugs" (PDF). Pharmaceutical and Medical Devices Agency. 2006.
- ↑ "The Drug Committee of Ministry of Health, Labour and Welfare in Japan has accepted a 2-year extension of market exclusivity of Lexapro®". GlobeNewswire News Room (Press release). 2018-04-27. Retrieved 2020-03-16.
- ↑ "Lundbeck Launches Antidepressent Lexapro In Japan". Asian Scientist Magazine. 2011-08-27. Retrieved 2018-09-16.
- ↑ Nomura S, Sawamura T, Kobayashi N, Yoshino A (Sep 2004). "Medication algorithm for mood disorders: Present status and future direction in Japan". International Journal of Psychiatry in Clinical Practice. 8 (3): 139–45. doi:10.1080/13651500410005432-1. PMID 24926843. S2CID 8210423.
- ↑ Otsubo T, Akimoto Y, Yamada H, et al. (Jan 2005). "A comparative study of the efficacy and safety profiles between fluvoxamine and nortriptyline in Japanese patients with major depression". Pharmacopsychiatry. 38 (1): 30–5. doi:10.1055/s-2005-837769. PMID 15706464. S2CID 25949175.
- ↑ Antidepressant Mirtazapine (Remeron) Submitted For Approval In Japan.
- ↑ "Meiji Seika Pharma Co., Ltd. | Meiji Holdings" . Retrieved 2018-09-16.
- ↑ "Pharmaceuticals | Meiji Holdings" . Retrieved 2018-09-16.
- ↑ "Meiji Seika Pharma Co., Ltd" (PDF). 2015.
- ↑ Products Approved in FY 2009: New Drugs. "List of Approved Products" (PDF). Pharmaceuticals and Medical Devices Agency.
{{cite web}}: CS1 maint: numeric names: authors list (link) - ↑ "Shionogi, Eli Lilly Japan to Comarket Cymbalta". PHARMA JAPAN. Retrieved 2020-03-16.
- ↑ "Cymbalta was approved today by MHLW for an additional indication of "pain associated with chronic back pain" (PDF).
- ↑ "Search results detail| Kusurino-Shiori(Drug information Sheet)". www.rad-ar.or.jp. Retrieved 2020-03-16.
- ↑ "Search results detail| Kusurino-Shiori(Drug information Sheet)". www.rad-ar.or.jp. Retrieved 2020-03-16.
- ↑ "approval for an additional indication of a serotonin/noradrenalin reuptake inhibitor, Cymbalta" (PDF).
- ↑ Fraende M (April 22, 2011). "Lundbeck gets Lexapro approval in Japan". Reuters.
- ↑ "Takeda and Lundbeck Submit New Drug Application (NDA) for Vortioxetine in Japan for the Treatment of Major Depressive Disorder (MDD)". www.takeda.com. Retrieved 2018-12-31.
- ↑ "Takeda and Lundbeck submit New Drug Application (NDA) for vortioxetine in Japan for the treatment of Major Depressive Disorder (MDD)". H. Lundbeck A/S. Retrieved 2018-12-31.