Related Research Articles

Lipolysis is the metabolic pathway through which lipid triglycerides are hydrolyzed into a glycerol and three fatty acids. It is used to mobilize stored energy during fasting or exercise, and usually occurs in fat adipocytes. The most important regulatory hormone in lipolysis is insulin; lipolysis can only occur when insulin action falls to low levels, as occurs during fasting. Other hormones that affect lipolysis include glucagon, epinephrine, norepinephrine, growth hormone, atrial natriuretic peptide, brain natriuretic peptide, and cortisol.

Brown adipose tissue (BAT) or brown fat makes up the adipose organ together with white adipose tissue. Brown adipose tissue is found in almost all mammals.

Aquaporins, also called water channels, are channel proteins from a larger family of major intrinsic proteins that form pores in the membrane of biological cells, mainly facilitating transport of water between cells. The cell membranes of a variety of different bacteria, fungi, animal and plant cells contain aquaporins through which water can flow more rapidly into and out of the cell than by diffusing through the phospholipid bilayer. Aquaporins have six membrane-spanning alpha helical domains with both carboxylic and amino terminals on the cytoplasmic side. Two hydrophobic loops contain conserved asparagine-proline-alanine which form a barrel surrounding a central pore-like region that contains additional protein density. Because aquaporins are usually always open and are prevalent in just about every cell type, this leads to a misconception that water readily passes through the cell membrane down its concentration gradient. Water can pass through the cell membrane through simple diffusion because it is a small molecule, and through osmosis, in cases where the concentration of water outside of the cell is greater than that of the inside. However, because water is a polar molecule this process of simple diffusion is relatively slow, and the majority of water passes through aquaporin.

Adipose tissue, body fat, or simply fat is a loose connective tissue composed mostly of adipocytes. In addition to adipocytes, adipose tissue contains the stromal vascular fraction (SVF) of cells including preadipocytes, fibroblasts, vascular endothelial cells and a variety of immune cells such as adipose tissue macrophages. Adipose tissue is derived from preadipocytes. Its main role is to store energy in the form of lipids, although it also cushions and insulates the body. Far from being hormonally inert, adipose tissue has, in recent years, been recognized as a major endocrine organ, as it produces hormones such as leptin, estrogen, resistin, and cytokines. The two types of adipose tissue are white adipose tissue (WAT), which stores energy, and brown adipose tissue (BAT), which generates body heat. The formation of adipose tissue appears to be controlled in part by the adipose gene. Adipose tissue – more specifically brown adipose tissue – was first identified by the Swiss naturalist Conrad Gessner in 1551.

Adipocytes, also known as lipocytes and fat cells, are the cells that primarily compose adipose tissue, specialized in storing energy as fat. Adipocytes are derived from mesenchymal stem cells which give rise to adipocytes through adipogenesis. In cell culture, adipocytes can also form osteoblasts, myocytes and other cell types.
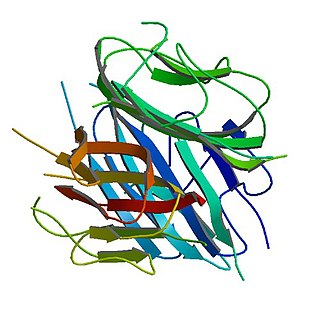
Adiponectin is a protein hormone and adipokine, which is involved in regulating glucose levels as well as fatty acid breakdown. In humans it is encoded by the ADIPOQ gene and it is produced in primarily in adipose tissue, but also in muscle, and even in the brain.

CD36, also known as platelet glycoprotein 4, fatty acid translocase (FAT), scavenger receptor class B member 3 (SCARB3), and glycoproteins 88 (GP88), IIIb (GPIIIB), or IV (GPIV) is a protein that in humans is encoded by the CD36 gene. The CD36 antigen is an integral membrane protein found on the surface of many cell types in vertebrate animals. It imports fatty acids inside cells and is a member of the class B scavenger receptor family of cell surface proteins. CD36 binds many ligands including collagen, thrombospondin, erythrocytes parasitized with Plasmodium falciparum, oxidized low density lipoprotein, native lipoproteins, oxidized phospholipids, and long-chain fatty acids.
Lipogenesis is the conversion of fatty acid and glycerol into fats OR metabolic process through which acetyl-CoA is converted to triglyceride for storage in fat. The triglycerides in fat are packaged within cytoplasmic lipid droplets. The process Lipogenesis encompasses both fatty acid and triglyceride synthesis, with the latter being the process by which fatty acids are esterified to glycerol before being packaged into very-low-density lipoprotein (VLDL). Fatty acids are produced in the cytoplasm of cells by repeatedly adding two-carbon units to acetyl-CoA. Triacyglycerol synthesis on the other hand, occurs in the endoplasmic reticulum membrane of cells by bonding three fatty acid molecules to a glycerol molecule. Both processes take place mainly in liver and adipose tissue. Nevertheless, it also occurs to some extent in other tissues such as the gut and kidney. A review on lipogenes in the brain was published in 2008 by Lopez and Vidal-Puig. After being packaged into VLDL in the liver, the resulting lipoprotein is then secreted directly into the blood for delivery to peripheral tissues.

Stearoyl-CoA desaturase (Δ-9-desaturase) is an endoplasmic reticulum enzyme that catalyzes the rate-limiting step in the formation of monounsaturated fatty acids (MUFAs), specifically oleate and palmitoleate from stearoyl-CoA and palmitoyl-CoA. Oleate and palmitoleate are major components of membrane phospholipids, cholesterol esters and alkyl-diacylglycerol. In humans, the enzyme is encoded by the SCD gene.

Perilipin, also known as lipid droplet-associated protein, Perilipin 1, or PLIN, is a protein that, in humans, is encoded by the PLIN gene. The perilipins are a family of proteins that associate with the surface of lipid droplets. Phosphorylation of perilipin is essential for the mobilization of fats in adipose tissue.
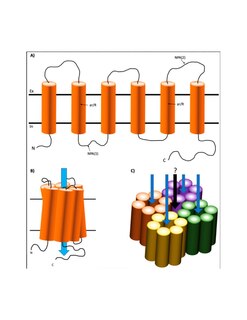
Aquaporin-4, also known as AQP4, is a water channel protein encoded by the AQP4 gene in humans. AQP4 belongs to the aquaporin family of integral membrane proteins that conduct water through the cell membrane. A limited number of aquaporins are found within the central nervous system (CNS): AQP1, 3, 4, 5, 8, 9, and 11, but more exclusive representation of AQP1, 4, and 9 are found in the brain and spinal cord. AQP4 shows the largest presence in the cerebellum and spinal cord grey matter. In the CNS, AQP4 is the most prevalent aquaporin channel, specifically located at the perimicrovessel astrocyte foot processes, glia limitans, and ependyma. In addition, this channel is commonly found facilitating water movement near cerebrospinal fluid and vasculature.

Aquaporin 3 is the protein product of the human AQP3 gene. It is found in the basolateral cell membrane of principal collecting duct cells and provides a pathway for water to exit these cells. Aquaporin 3 is also permeable to glycerol, ammonia, urea, and hydrogen peroxide. It is expressed in various tissues including the skin, respiratory tract, and kidneys as well as various types of cancers. In the kidney, aquaproin 3 is unresponsive to vasopressin, unlike Aquaporin 2. This protein is also a determinant for the GIL blood group system.

Aquaporin 1 is a protein that in humans is encoded by the AQP1 gene.
The ERRs are orphan nuclear receptors, meaning the identity of their endogenous ligand has yet to be unambiguously determined. They are named because of sequence homology with estrogen receptors, but do not appear to bind estrogens or other tested steroid hormones.

Glycerol-3-phosphate dehydrogenase (GPDH) is an enzyme that catalyzes the reversible redox conversion of dihydroxyacetone phosphate to sn-glycerol 3-phosphate.
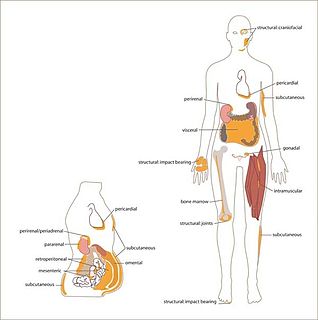
White adipose tissue (WAT) or white fat is one of the two types of adipose tissue found in mammals. The other kind is brown adipose tissue. It is composed of monolocular adipocytes.
Major intrinsic proteins comprise a large superfamily of transmembrane protein channels that are grouped together on the basis of homology. The MIP superfamily includes three subfamilies: aquaporins, aquaglyceroporins and S-aquaporins.
- The aquaporins (AQPs) are water selective.
- The aquaglyceroporins are permeable to water, but also to other small uncharged molecules such as glycerol.
- The third subfamily, with little conserved amino acid sequences around the NPA boxes, include 'superaquaporins' (S-aquaporins).

Adipose triglyceride lipase, also known as patatin-like phospholipase domain-containing protein 2 and ATGL, is an enzyme that in humans is encoded by the PNPLA2 gene. ATGL catalyses the first reaction of lipolysis, where triacylglycerols are hydrolysed to diacylglycerols.

Aquaporin-9 is a protein that in humans is encoded by the AQP9 gene.

Aquaporin-7 is a protein that in humans is encoded by the AQP7 gene.
References
- 1 2 Madeira, Ana; Moura, Teresa F.; Soveral, Graça (2015-02-01). "Aquaglyceroporins: implications in adipose biology and obesity". Cellular and Molecular Life Sciences. 72 (4): 759–771. doi:10.1007/s00018-014-1773-2. ISSN 1420-682X. PMID 25359234. S2CID 14789298.
- ↑ Bhattacharjee, Hiranmoy; Mukhopadhyay, Rita; Thiyagarajan, Saravanamuthu; Rosen, Barry P. (2008-09-01). "Aquaglyceroporins: ancient channels for metalloids". Journal of Biology. 7 (9): 33. doi:10.1186/jbiol91. ISSN 1475-4924. PMC 2776386 . PMID 19014407.
- ↑ Liu, Yangjian; Promeneur, Dominique; Rojek, Aleksandra; Kumar, Nirbhay; Frøkiær, Jørgen; Nielsen, Søren; King, Landon S.; Agre, Peter; Carbrey, Jennifer M. (2007-07-24). "Aquaporin 9 is the major pathway for glycerol uptake by mouse erythrocytes, with implications for malarial virulence". Proceedings of the National Academy of Sciences of the United States of America. 104 (30): 12560–12564. Bibcode:2007PNAS..10412560L. doi: 10.1073/pnas.0705313104 . ISSN 0027-8424. PMC 1941508 . PMID 17636116.