Related Research Articles

An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit. Electrodes are essential parts of batteries that can consist of a variety of materials depending on the type of battery.

A lithium-ion or Li-ion battery is a type of rechargeable battery that uses the reversible intercalation of Li+ ions into electronically conducting solids to store energy. In comparison with other commercial rechargeable batteries, Li-ion batteries are characterized by higher specific energy, higher energy density, higher energy efficiency, a longer cycle life, and a longer calendar life. Also noteworthy is a dramatic improvement in lithium-ion battery properties after their market introduction in 1991: within the next 30 years, their volumetric energy density increased threefold while their cost dropped tenfold.
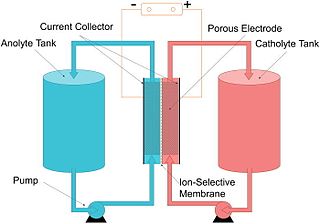
A flow battery, or redox flow battery, is a type of electrochemical cell where chemical energy is provided by two chemical components dissolved in liquids that are pumped through the system on separate sides of a membrane. Ion transfer inside the cell occurs through the membrane while both liquids circulate in their own respective space. Cell voltage is chemically determined by the Nernst equation and ranges, in practical applications, from 1.0 to 2.43 volts. The energy capacity is a function of the electrolyte volume and the power is a function of the surface area of the electrodes.
The electrochemical window (EW) of a substance is the electrode electric potential range between which the substance is neither oxidized nor reduced. The EW is one of the most important characteristics to be identified for solvents and electrolytes used in electrochemical applications. The EW is a term that is commonly used to indicate the potential range and the potential difference. It is calculated by subtracting the reduction potential from the oxidation potential.
A polymer-based battery uses organic materials instead of bulk metals to form a battery. Currently accepted metal-based batteries pose many challenges due to limited resources, negative environmental impact, and the approaching limit of progress. Redox active polymers are attractive options for electrodes in batteries due to their synthetic availability, high-capacity, flexibility, light weight, low cost, and low toxicity. Recent studies have explored how to increase efficiency and reduce challenges to push polymeric active materials further towards practicality in batteries. Many types of polymers are being explored, including conductive, non-conductive, and radical polymers. Batteries with a combination of electrodes are easier to test and compare to current metal-based batteries, however batteries with both a polymer cathode and anode are also a current research focus. Polymer-based batteries, including metal/polymer electrode combinations, should be distinguished from metal-polymer batteries, such as a lithium polymer battery, which most often involve a polymeric electrolyte, as opposed to polymeric active materials.

A lithium-ion capacitor is a hybrid type of capacitor classified as a type of supercapacitor. It is called a hybrid because the anode is the same as those used in lithium-ion batteries and the cathode is the same as those used in supercapacitors. Activated carbon is typically used as the cathode. The anode of the LIC consists of carbon material which is often pre-doped with lithium ions. This pre-doping process lowers the potential of the anode and allows a relatively high output voltage compared to other supercapacitors.
Rechargeable lithium metal batteries are secondary lithium metal batteries. They have metallic lithium as a negative electrode, sometimes referred to as the battery anode. The high specific capacity of lithium metal, very low redox potential and low density make it the ideal anode material for high energy density battery technologies. Rechargeable lithium metal batteries can have a long run time due to the high charge density of lithium. Several companies and many academic research groups are currently researching and developing rechargeable lithium metal batteries as they are considered a leading pathway for development beyond lithium-ion batteries. Some rechargeable lithium metal batteries employ a liquid electrolyte and some employ a solid-state electrolyte.
The lithium–air battery (Li–air) is a metal–air electrochemical cell or battery chemistry that uses oxidation of lithium at the anode and reduction of oxygen at the cathode to induce a current flow.
A metal–air electrochemical cell is an electrochemical cell that uses an anode made from pure metal and an external cathode of ambient air, typically with an aqueous or aprotic electrolyte.
A potassium-ion battery or K-ion battery is a type of battery and analogue to lithium-ion batteries, using potassium ions for charge transfer instead of lithium ions. It was invented by the Iranian/American chemist Ali Eftekhari in 2004.

Sodium-ion batteries (NIBs, SIBs, or Na-ion batteries) are several types of rechargeable batteries, which use sodium ions (Na+) as its charge carriers. In some cases, its working principle and cell construction are similar to those of lithium-ion battery (LIB) types, but it replaces lithium with sodium as the intercalating ion. Sodium belongs to the same group in the periodic table as lithium and thus has similar chemical properties. Although, in some cases (such as aqueous Na-ion batteries) they are quite different from Li-ion batteries.

A supercapacitor (SC), also called an ultracapacitor, is a high-capacity capacitor, with a capacitance value much higher than solid-state capacitors but with lower voltage limits. It bridges the gap between electrolytic capacitors and rechargeable batteries. It typically stores 10 to 100 times more energy per unit volume or mass than electrolytic capacitors, can accept and deliver charge much faster than batteries, and tolerates many more charge and discharge cycles than rechargeable batteries.
Aluminium-ion batteries are a class of rechargeable battery in which aluminium ions serve as charge carriers. Aluminium can exchange three electrons per ion. This means that insertion of one Al3+ is equivalent to three Li+ ions. Thus, since the ionic radii of Al3+ (0.54 Å) and Li+ (0.76 Å) are similar, significantly higher numbers of electrons and Al3+ ions can be accepted by cathodes with little damage. Al has 50 times (23.5 megawatt-hours m-3) the energy density of Li and is even higher than coal.
A lithium-ion flow battery is a flow battery that uses a form of lightweight lithium as its charge carrier. The flow battery stores energy separately from its system for discharging. The amount of energy it can store is determined by tank size; its power density is determined by the size of the reaction chamber.
Research in lithium-ion batteries has produced many proposed refinements of lithium-ion batteries. Areas of research interest have focused on improving energy density, safety, rate capability, cycle durability, flexibility, and cost.

NASICON is an acronym for sodium (Na) super ionic conductor, which usually refers to a family of solids with the chemical formula Na1+xZr2SixP3−xO12, 0 < x < 3. In a broader sense, it is also used for similar compounds where Na, Zr and/or Si are replaced by isovalent elements. NASICON compounds have high ionic conductivities, on the order of 10−3 S/cm, which rival those of liquid electrolytes. They are caused by hopping of Na ions among interstitial sites of the NASICON crystal lattice.
Lithium–silicon battery is a name used for a subclass of lithium-ion battery technology that employs a silicon-based anode and lithium ions as the charge carriers. Silicon based materials generally have a much larger specific capacity, for example 3600 mAh/g for pristine silicon, relative to graphite, which is limited to a maximum theoretical capacity of 372 mAh/g for the fully lithiated state LiC6. Silicon's large volume change (approximately 400% based on crystallographic densities) when lithium is inserted, along with high reactivity in the charged state, are obstacles to commercializing this type of anode. Commercial battery anodes may have small amounts of silicon, boosting their performance slightly. The amounts are closely held trade secrets, limited as of 2018 to at most 10% of the anode. Lithium-silicon batteries also include cell configurations where Si is in compounds that may at low voltage store lithium by a displacement reaction, including silicon oxycarbide, silicon monoxide or silicon nitride.
Magnesium batteries are batteries that utilize magnesium cations as charge carriers and possibly in the anode in electrochemical cells. Both non-rechargeable primary cell and rechargeable secondary cell chemistries have been investigated. Magnesium primary cell batteries have been commercialised and have found use as reserve and general use batteries.
A zinc-ion battery or Zn-ion battery (abbreviated as ZIB) uses zinc ions (Zn2+) as the charge carriers. Specifically, ZIBs utilize Zn as the anode, Zn-intercalating materials as the cathode, and a Zn-containing electrolyte. Generally, the term zinc-ion battery is reserved for rechargeable (secondary) batteries, which are sometimes also referred to as rechargeable zinc metal batteries (RZMB). Thus, ZIBs are different than non-rechargeable (primary) batteries which use zinc, such as alkaline or zinc–carbon batteries.
Superconcentrated electrolytes, also known as water-in-salt or solvent-in-salt liquids, usually refer to chemical systems, which are liquid near room temperature and consist of a solvent-to-dissoved salt in a molar ratio near or smaller than ca. 4-8, i.e. where all solvent molecules are coordinated to cations, and no free solvent molecules remain. Since ca. 2010 such liquid electrolytes found several applications, primarily for batteries. In the case of lithium metal batteries and lithium-ion batteries most commonly used anions for superconcentrated electrolytes are those, that are large, asymmetric and rotationally-vibrationally flexible, such bis(trifluoromethanesulfonyl)amide and bis(fluorosulfonyl)amide. Noteworthy, lithium chloride and sodium perchlorate also form water-in-salt solutions.
References
- 1 2 Malik, Rahul (September 2017). "Aqueous Li-Ion Batteries: Now in Striking Distance". Joule. 1 (1): 17–19. doi: 10.1016/j.joule.2017.08.016 .
- 1 2 "UMD & Army Researchers Discover Salty Solution to Better, Safer Batteries". www.batterypoweronline.com. December 2, 2015. Retrieved 2018-07-10.
- 1 2 3 4 5 Suo, L.; Borodin, O.; Gao, T.; Olguin, M.; Ho, J.; Fan, X.; Luo, C.; Wang, C.; Xu, K. (2015). "'Water-in-salt' electrolyte enables high-voltage aqueous lithium-ion chemistries". Science. 350 (6263): 938–943. doi:10.1126/science.aab1595. PMID 26586759. S2CID 206637574.
- "Water-based lithium-ion batteries without explosive risks now a reality". Phys.org. September 6, 2017.
- ↑ Liu, Jilei; Xu, Chaohe; Chen, Zhen; Ni, Shibing; Shen, Ze Xiang (January 2018). "Progress in aqueous rechargeable batteries". Green Energy & Environment. 3 (1): 20–41. doi: 10.1016/j.gee.2017.10.001 .
- ↑ Xu, Kang; Wang, Chunsheng (6 October 2016). "Batteries: Widening voltage windows". Nature Energy. 1 (10): 16161. Bibcode:2016NatEn...116161X. doi:10.1038/nenergy.2016.161. S2CID 100576016.
- 1 2 3 Hopkins, Gina (November 16, 2017). "Watch: Cuts and dunks don't stop new lithium-ion battery - Futurity". Futurity. Retrieved 2018-07-10.
- ↑ Yang, Chongyin; Chen, Ji; Qing, Tingting; Fan, Xiulin; Sun, Wei; von Cresce, Arthur; Ding, Michael S.; Borodin, Oleg; Vatamanu, Jenel; Schroeder, Marshall A.; Eidson, Nico; Wang, Chunsheng; Xu, Kang (September 2017). "4.0 V Aqueous Li-Ion Batteries". Joule. 1 (1): 122–132. doi: 10.1016/j.joule.2017.08.009 .
- 1 2 3 4 Schelmetic, Tracey (September 22, 2017). "UMD and U.S. Army Research Lab Engineers Develop 4.0 Aqueous Lithium-Ion Battery". Design News. Retrieved 2018-07-10.
- 1 2 Sui, Yiming; Ji, Xiulei (2021-06-09). "Anticatalytic Strategies to Suppress Water Electrolysis in Aqueous Batteries". Chemical Reviews. 121 (11): 6654–6695. doi:10.1021/acs.chemrev.1c00191. ISSN 0009-2665. PMID 33900728. S2CID 233409171.