Related Research Articles
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and compounds made of atoms, molecules and ions: their composition, structure, properties, behavior and the changes they undergo during reactions with other substances. Chemistry also addresses the nature of chemical bonds in chemical compounds.
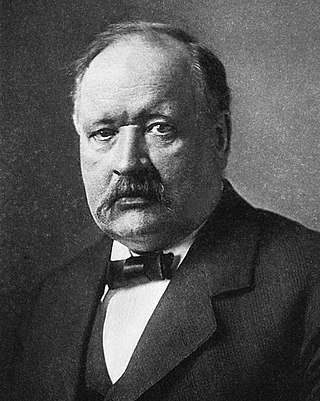
Svante August Arrhenius was a Swedish scientist. Originally a physicist, but often referred to as a chemist, Arrhenius was one of the founders of the science of physical chemistry. He received the Nobel Prize for Chemistry in 1903, becoming the first Swedish Nobel laureate. In 1905, he became the director of the Nobel Institute, where he remained until his death.

In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (Ea) of a reaction is measured in kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). Activation energy can be thought of as the magnitude of the potential barrier (sometimes called the energy barrier) separating minima of the potential energy surface pertaining to the initial and final thermodynamic state. For a chemical reaction to proceed at a reasonable rate, the temperature of the system should be high enough such that there exists an appreciable number of molecules with translational energy equal to or greater than the activation energy. The term "activation energy" was introduced in 1889 by the Swedish scientist Svante Arrhenius.

Jacobus Henricus van 't Hoff Jr. was a Dutch physical chemist. A highly influential theoretical chemist of his time, van 't Hoff was the first winner of the Nobel Prize in Chemistry. His pioneering work helped found the modern theory of chemical affinity, chemical equilibrium, chemical kinetics, and chemical thermodynamics. In his 1874 pamphlet, van 't Hoff formulated the theory of the tetrahedral carbon atom and laid the foundations of stereochemistry. In 1875, he predicted the correct structures of allenes and cumulenes as well as their axial chirality. He is also widely considered one of the founders of physical chemistry as the discipline is known today.
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in 1884 that the van 't Hoff equation for the temperature dependence of equilibrium constants suggests such a formula for the rates of both forward and reverse reactions. This equation has a vast and important application in determining the rate of chemical reactions and for calculation of energy of activation. Arrhenius provided a physical justification and interpretation for the formula. Currently, it is best seen as an empirical relationship. It can be used to model the temperature variation of diffusion coefficients, population of crystal vacancies, creep rates, and many other thermally induced processes and reactions. The Eyring equation, developed in 1935, also expresses the relationship between rate and energy.

George Charles de Hevesy was a Hungarian radiochemist and Nobel Prize in Chemistry laureate, recognized in 1943 for his key role in the development of radioactive tracers to study chemical processes such as in the metabolism of animals. He also co-discovered the element hafnium.

Per Teodor Cleve was a Swedish chemist, biologist, mineralogist and oceanographer. He is best known for his discovery of the chemical elements holmium and thulium.

The Royal Society of Chemistry (RSC) is a learned society and professional association in the United Kingdom with the goal of "advancing the chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 34,000 in the UK and a further 8,000 abroad.

Archer John Porter Martin was a British chemist who shared the 1952 Nobel Prize in Chemistry for the invention of partition chromatography with Richard Synge.

Ernst Julius Cohen ForMemRS was a Dutch Jewish chemist known for his work on the allotropy of metals. Cohen studied chemistry under Svante Arrhenius in Stockholm, Henri Moissan at Paris, and Jacobus van't Hoff at Amsterdam. In 1893 he became Van't Hoff's assistant and in 1902 he became professor of Physical Chemistry at the University of Utrecht, a position which he held until his retirement in 1939. Throughout his life, Cohen studied the allotropy of tin. Cohen's areas of research included polymorphism of both elements and compounds, photographic chemistry, electrochemistry, pizeochemistry, and the history of science. He published more than 400 papers and numerous books.
Sir Hugh Stott Taylor was an English chemist primarily interested in catalysis. In 1925, in a landmark contribution to catalytic theory, Taylor suggested that a catalysed chemical reaction is not catalysed over the entire solid surface of the catalyst but only at certain 'active sites' or centres. He also developed important methods for procuring heavy water during World War II and pioneered the use of stable isotopes in studying chemical reactions.

The Nobel Committee for Physics is the Nobel Committee responsible for proposing laureates for the Nobel Prize for Physics. The Nobel Committee for Physics is appointed by the Royal Swedish Academy of Sciences. It usually consists of Swedish professors of physics who are members of the Academy, although the Academy in principle could appoint anyone to the Committee.
Ludwig Ferdinand Wilhelmy was a German scientist who is usually credited with publishing the first quantitative study in chemical kinetics.

Carl Axel Arrhenius was a Swedish military officer, amateur geologist, and chemist. He is best known for his discovery of the mineral ytterbite in 1787.
Jerker Porath, was a Swedish biochemist who invented several separation methods for biomolecules. He was born in Sala.
Chicita Frances Culberson was an American lichenologist.
John Calvin Giddings was a Distinguished Professor of chemistry at the University of Utah. Giddings received a B.S. degree from Brigham Young University in 1952 and a PhD from the University of Utah in 1954. Following postdoctoral work at the University of Utah and the University of Wisconsin, he joined the faculty of the University of Utah as assistant professor of chemistry in 1957. He became associate professor in 1959, research professor in 1962, and professor in 1966. Giddings authored or co-authored more than 400 publications and edited 32 books in the field of chemistry. He was executive editor of the journal Separation Science and Technology, and the editor of the series Advances in chromatography.

Klaus Hermann Mosbach was a Swedish applied biochemist based at Lund University. He founded the Center for Molecular Imprinting in Lund, Sweden and was co-founder of the Institute of biotechnology at ETH Zurich Switzerland 1982. He was a great visionary who gave shape to the modern era of Molecular imprinting for which he was awarded the plaque at the international meeting of molecular imprinting in 2010 in New Orleans, United States.

Pernilla Wittung-Stafshede is a Swedish biophysical chemist, born in 1968, who is a professor of chemical biology at Chalmers University of Technology in Gothenburg. In 2019 she was named by International Union of Pure and Applied Chemistry as a Distinguished Woman in Chemistry.
References
- ↑ Koester, Vera; Goedecke, Catharina (2023). "Physical Chemistry Pioneer: Svante Arrhenius (1859 – 1927)". ChemViews. doi:10.1002/chemv.202300014.
- ↑ "Arrhenius-plaketten". The Swedish Chemical Society. Retrieved 31 October 2024.
- ↑ "The Swedish Chemical Society - Arrhenius Plaque | EFG - European Funding Guide". www.european-funding-guide.eu. Retrieved 2024-11-06.
- ↑ "The M.S. Tswett Chromatography Medal 1979". Chromatographia. 12 (10): 679. October 1979. doi:10.1007/BF02302945.
- ↑ Ettre, L.S.; Zlatkis, A. (1 January 1979). "Per Flodin". Journal of Chromatography Library. 17: 67–74. doi:10.1016/S0301-4770(08)60636-3. ISBN 978-0-444-41754-1.
- ↑ Dėdinaitė, Andra; Claesson, Per M. (2017). "Synergies in lubrication". Physical Chemistry Chemical Physics. 19 (35): 23677–23689. doi: 10.1039/C7CP03517A . PMID 28681896.
- ↑ "Professor Lisbeth Olsson receives the Arrhenius plaque". 2 January 2019. Retrieved 31 October 2024.
- ↑ "Arrhenius-plaketten till kemiprofessor Berit Olofsson".