This article includes a list of references, related reading, or external links, but its sources remain unclear because it lacks inline citations .(May 2019) |
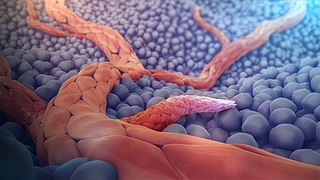
Arteriogenesis refers to an increase in the diameter of existing arterial vessels.
This article includes a list of references, related reading, or external links, but its sources remain unclear because it lacks inline citations .(May 2019) |
Arteriogenesis refers to an increase in the diameter of existing arterial vessels.
Mechanically, arteriogenesis is linked to elevated pressure, which increases radial wall stress, and elevated flow, which increases endothelial surface stress. The vessel increases in diameter until the stress is normalized (Prior et al., 2004). Arteriogenesis does not occur every time there is an increase in flow, however. Most vessel networks can handle increased flow without significantly increasing diameter because flow increases with the square (power 2) of the vessel diameter. Initial experiments demonstrated this phenomenon in that mature vessels are unlikely to respond to increased flow by increasing diameter, but will respond to decreased flow by decreasing diameter (Brownlee & Langille, 1991). Another experiment showed that increasing shear stress caused an immediate increase in vessel expansion followed by a rapid decrease, as well as demonstrating that the mature vessels do indeed respond more favorably to decreased stress (Tuttle et al., 2001).
Chemically, arteriogenesis is related to upregulation of cytokines and cell adhesion receptors. More specifically, mechanical stresses cause endothelial cells to produce chemical facilitators that begin the process of increasing diameter. An increase in shear stress causes an increase in the number of monocyte chemoattractant protein-1 (MCP-1) molecules expressed on the surface of vessel walls as well as increased levels of TNF-α, bFGF, and MMP. MCP-1 increases the tendency of monocytes to attach to the cell wall. TNF-α provides an inflammatory environment for the cells to develop while bFGF helps induce mitosis in the endothelial cells. Finally, MMPs remodel the space around the artery to provide the space for expansion (Van Royen et al., 2001). Another potent chemical signal is nitric oxide (NO), demonstrated to be a major factor in increasing vessel diameter in response to increased flow until the shear stress is restored to the normal level (Tronc et al., 1996).
bFGF is known to increase both arteriogenesis and angiogenesis in vivo. However, it is not sufficient as a monotherapy to increase arteriogenesis. In a placebo study determining the effects of bFGF on arteriogenesis, patients were treated with one bolus of bFGF. The treatment helped reduce anginal symptoms but did not significantly affect arteriogenesis. Thus, it is speculated that other growth factors work in tandem with bFGF to produce the desired response and that growth factors must be administered at varying time points throughout the duration of the experiment (Van Royen et al., 2001). This finding is important because it shows that arteriogenesis is the result of a combination of signaling cascades and growth factors as opposed to being tied to a single chemical.
MCP1 (now called CCL2) is especially important in arteriogenesis. Since MCP-1 attracts monocytes it can produce an immune cascade to aid inflammation. Monocytes can enter the vessel wall to become macrophages and produce inflammatory cytokines such as TNF-α in addition to aiding the production of bFGF and MMP (Van Royen et al., 2000). Macrophages also produce vascular endothelial growth factor (VEGF) that is a huge contributor to the growth signaling of endothelial cells. Endothelial cells have a receptor devoted to VEGF aptly named VEGF receptor-1 that immediately signals rapid mitosis in the cells (Prior et al., 2004). One study showed that local infusion of MCP-1 caused a large increase in conductance in both collateral and peripheral vessels while diminished levels of MCP-1 hindered the process of arteriogenesis (Ito et al., 1997). This indicates that monocytes play a significant role in inducing arteriogenesis.
Poiseuille’s Law for flow indicates that the total flow in a tube is related to the diameter of the tube by a power of four. Thus, an increase in the diameter of a high order blood vessel such as an arteriole vastly increases the total flow that a given vessel network can withstand. This flow increase is vitally important in the microvasculature remodeling following exercise, especially in sprint training. Sprint training is a type of anaerobic exercise that relies on having the maximum amount of blood available to the vessel network at any given time (Prior et al., 2004).
Arteriogenesis also has much in common with the mechanisms of atherosclerosis. Monocytes invade the endothelial tissue, inflammatory cytokines are released, endothelial cells proliferate into the surrounding tissue, and cell adhesion receptors are upregulated. Presently, the effects of arteriogenesis on atherosclerosis are unknown, although MCP-1 receptors are known to be associated with plaque formation (Van Royen et al., 2001).
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, formed in the earlier stage of vasculogenesis. Angiogenesis continues the growth of the vasculature mainly by processes of sprouting and splitting, but processes such as coalescent angiogenesis, vessel elongation and vessel cooption also play a role. Vasculogenesis is the embryonic formation of endothelial cells from mesoderm cell precursors, and from neovascularization, although discussions are not always precise. The first vessels in the developing embryo form through vasculogenesis, after which angiogenesis is responsible for most, if not all, blood vessel growth during development and in disease.

Tumor necrosis factor is a cytokine and member of the TNF superfamily, which consists of various transmembrane proteins with a homologous TNF domain. It is the first cytokine to be described as an adipokine as secreted by adipose tissue.
A vulnerable plaque is a kind of atheromatous plaque – a collection of white blood cells and lipids in the wall of an artery – that is particularly unstable and prone to produce sudden major problems such as a heart attack or stroke.
Vascular endothelial growth factor, originally known as vascular permeability factor (VPF), is a signal protein produced by many cells that stimulates the formation of blood vessels. To be specific, VEGF is a sub-family of growth factors, the platelet-derived growth factor family of cystine-knot growth factors. They are important signaling proteins involved in both vasculogenesis and angiogenesis.

The chemokine ligand 2 (CCL2) is also referred to as monocyte chemoattractant protein 1 (MCP1) and small inducible cytokine A2. CCL2 is a small cytokine that belongs to the CC chemokine family. CCL2 tightly regulates cellular mechanics and thereby recruits monocytes, memory T cells, and dendritic cells to the sites of inflammation produced by either tissue injury or infection.
In medicine, collateralization, also vessel collateralization and blood vessel collateralization, is the growth of a blood vessel or several blood vessels that serve the same end organ or vascular bed as another blood vessel that cannot adequately supply that end organ or vascular bed sufficiently.

E-selectin, also known as CD62 antigen-like family member E (CD62E), endothelial-leukocyte adhesion molecule 1 (ELAM-1), or leukocyte-endothelial cell adhesion molecule 2 (LECAM2), is a selectin cell adhesion molecule expressed only on endothelial cells activated by cytokines. Like other selectins, it plays an important part in inflammation. In humans, E-selectin is encoded by the SELE gene.

Interleukin 20 (IL20) is a protein that is in humans encoded by the IL20 gene which is located in close proximity to the IL-10 gene on the 1q32 chromosome. IL-20 is a part of an IL-20 subfamily which is a part of a larger IL-10 family.

Interleukin 19 (IL-19) is an immunosuppressive protein that belongs to the IL-10 cytokine subfamily.

Chemokine ligand 7 (CCL7) is a small cytokine that was previously called monocyte-chemotactic protein 3 (MCP3). CCL7 is a small protein that belongs to the CC chemokine family and is most closely related to CCL2.
Chemokine ligand 13 (CCL13) is a small cytokine belonging to the CC chemokine family. Its gene is located on human chromosome 17 within a large cluster of other CC chemokines. CCL13 induces chemotaxis in monocytes, eosinophils, T lymphocytes, and basophils by binding cell surface G-protein linked chemokine receptors such as CCR2, CCR3 and CCR5. Activity of this chemokine has been implicated in allergic reactions such as asthma. CCL13 can be induced by the inflammatory cytokines interleukin-1 and TNF-α.

VEGF receptors (VEGFRs) are receptors for vascular endothelial growth factor (VEGF). There are three main subtypes of VEGFR, numbered 1, 2 and 3. Depending on alternative splicing, they may be membrane-bound (mbVEGFR) or soluble (sVEGFR).

Leukocyte extravasation is the movement of leukocytes out of the circulatory system and towards the site of tissue damage or infection. This process forms part of the innate immune response, involving the recruitment of non-specific leukocytes. Monocytes also use this process in the absence of infection or tissue damage during their development into macrophages.

72 kDa type IV collagenase also known as matrix metalloproteinase-2 (MMP-2) and gelatinase A is an enzyme that in humans is encoded by the MMP2 gene. The MMP2 gene is located on chromosome 16 at position 12.2.
Vascular endothelial growth factor B also known as VEGF-B is a protein that, in humans, is encoded by the VEGF-B gene. VEGF-B is a growth factor that belongs to the vascular endothelial growth factor family, of which VEGF-A is the best-known member.

Vascular endothelial growth factor A (VEGF-A) is a protein that in humans is encoded by the VEGFA gene.
Pancreatic stellate cells (PaSCs) are classified as myofibroblast-like cells that are located in exocrine regions of the pancreas. PaSCs are mediated by paracrine and autocrine stimuli and share similarities with the hepatic stellate cell. Pancreatic stellate cell activation and expression of matrix molecules constitute the complex process that induces pancreatic fibrosis. Synthesis, deposition, maturation and remodelling of the fibrous connective tissue can be protective, however when persistent it impedes regular pancreatic function.
Angiogenesis is the process of forming new blood vessels from existing blood vessels, formed in vasculogenesis. It is a highly complex process involving extensive interplay between cells, soluble factors, and the extracellular matrix (ECM). Angiogenesis is critical during normal physiological development, but it also occurs in adults during inflammation, wound healing, ischemia, and in pathological conditions such as rheumatoid arthritis, hemangioma, and tumor growth. Proteolysis has been indicated as one of the first and most sustained activities involved in the formation of new blood vessels. Numerous proteases including matrix metalloproteinases (MMPs), a disintegrin and metalloproteinase domain (ADAM), a disintegrin and metalloproteinase domain with throbospondin motifs (ADAMTS), and cysteine and serine proteases are involved in angiogenesis. This article focuses on the important and diverse roles that these proteases play in the regulation of angiogenesis.

Vascular remodelling is a process which occurs when an immature heart begins contracting, pushing fluid through the early vasculature. The process typically begins at day 22, and continues to the tenth week of human embryogenesis. This first passage of fluid initiates a signal cascade and cell movement based on physical cues including shear stress and circumferential stress, which is necessary for the remodelling of the vascular network, arterial-venous identity, angiogenesis, and the regulation of genes through mechanotransduction. This embryonic process is necessary for the future stability of the mature vascular network.
Endothelial activation is a proinflammatory and procoagulant state of the endothelial cells lining the lumen of blood vessels. It is most characterized by an increase in interactions with white blood cells (leukocytes), and it is associated with the early states of atherosclerosis and sepsis, among others. It is also implicated in the formation of deep vein thrombosis. As a result of activation, enthothelium releases Weibel–Palade bodies.