Related Research Articles

STS-50 was a NASA Space Shuttle mission, the 12th mission of the Columbia orbiter. Columbia landed at Kennedy Space Center for the first time ever due to bad weather at Edwards Air Force Base caused by the remnants of Hurricane Darby.
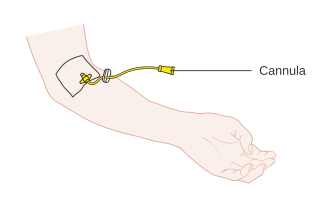
A cannula is a tube that can be inserted into the body, often for the delivery or removal of fluid or for the gathering of samples. In simple terms, a cannula can surround the inner or outer surfaces of a trocar needle thus extending the effective needle length by at least half the length of the original needle. Its size mainly ranges from 14 to 24 gauge. Different-sized cannula have different colours as coded.

Sterilization refers to any process that removes, kills, or deactivates all forms of life and other biological agents such as prions present in or on a specific surface, object, or fluid. Sterilization can be achieved through various means, including heat, chemicals, irradiation, high pressure, and filtration. Sterilization is distinct from disinfection, sanitization, and pasteurization, in that those methods reduce rather than eliminate all forms of life and biological agents present. After sterilization, an object is referred to as being sterile or aseptic.

A bioreactor refers to any manufactured device or system that supports a biologically active environment. In one case, a bioreactor is a vessel in which a chemical process is carried out which involves organisms or biochemically active substances derived from such organisms. This process can either be aerobic or anaerobic. These bioreactors are commonly cylindrical, ranging in size from litres to cubic metres, and are often made of stainless steel. It may also refer to a device or system designed to grow cells or tissues in the context of cell culture. These devices are being developed for use in tissue engineering or biochemical/bioprocess engineering.

Blow-Fill-Seal, also spelled as Blow/Fill/Seal, in this article abbreviated as BFS, is an automated manufacturing process by which plastic containers, such as bottles or ampoules are, in a continuous operation, blow-formed, filled, and sealed. It takes place in a sterile, enclosed area inside a machine, without human intervention, and thus can be used to aseptically manufacture sterile pharmaceutical or non-pharamceutical liquid/semiliquid unit-dosage forms. BFS is an advanced aseptic processing technology that is typically used for filling and packaging of certain sterile liquid formulations like liquid ophtalmics, inhalational anesthetics, or lavaging agents, but can also be used for injectables, parenterals, and several other liquid or semiliquid medications, with fill volumes ranging from 0.1...1000 cm³. Compared against traditional glass ampoules, BFS ampoules are inexpensive, lightweight, and shatterproof. Important BFS system suppliers are Weiler Engineering, Rommelag (Bottelpack), and Brevetti Angela.
A bioprocess is a specific process that uses complete living cells or their components to obtain desired products.
A biopharmaceutical, also known as a biologic(al) medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, whole blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living medicines used in cell therapy. Biologics can be composed of sugars, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. They are isolated from living sources—human, animal, plant, fungal, or microbial. They can be used in both human and animal medicine.

Clean-in-place (CIP) is a method of automated cleaning the interior surfaces of pipes, vessels, equipments, filters and associated fittings, without major disassembly. CIP is commonly used for equipment such as piping, tanks, and fillers. CIP employs turbulent flow through piping, or sprayballs for large surfaces. In some cases, CIP can also be accomplished with fill, soak and agitate.

A photobioreactor (PBR) refers to any cultivation system designed for growing photoautotrophic organisms using artificial light sources or solar light to facilitate photosynthesis. PBRs are typically used to cultivate microalgae, cyanobacteria, macroalgae, and some mosses. PBRs can be open systems, such as raceway ponds, which rely upon natural sources of light and carbon dioxide. Closed PBRs are flexible systems that can be controlled to the physiological requirements of the cultured organism, resulting in optimal growth rates and purity levels. PBRs are typically used for the cultivation of bioactive compounds for biofuels, pharmaceuticals, and other industrial uses.
Dissolved gas analysis (DGA) is an examination of electrical transformer oil contaminants. Insulating materials within electrical equipment liberate gases as they slowly break down over time. The composition and distribution of these dissolved gases are indicators of the effects of deterioration, such as pyrolysis or partial discharge, and the rate of gas generation indicates the severity. DGA is beneficial to a preventive maintenance program.
Merck Millipore was the brand used by Merck Group's global life science business until 2015 when the company re-branded. It was formed when Merck acquired the Millipore Corporation in 2010. Merck is a supplier to the life science industry. The Millipore Corporation was founded in 1954, and listed among the S&P 500 since the early 1990s, as an international biosciences company which makes micrometer pore-size filters and tests. In 2015, Merck acquired Sigma-Aldrich and merged it with Merck Millipore. In the United States and Canada, the life science business is now known as MilliporeSigma.
Aseptic processing is a processing technique wherein commercially thermally sterilized liquid products are packaged into previously sterilized containers under sterile conditions to produce shelf-stable products that do not need refrigeration. Aseptic processing has almost completely replaced in-container sterilization of liquid foods, including milk, fruit juices and concentrates, cream, yogurt, salad dressing, liquid egg, and ice cream mix. There has been an increasing popularity for foods that contain small discrete particles, such as cottage cheese, baby foods, tomato products, fruit and vegetables, soups, and rice desserts.
Air-free techniques refer to a range of manipulations in the chemistry laboratory for the handling of compounds that are air-sensitive. These techniques prevent the compounds from reacting with components of air, usually water and oxygen; less commonly carbon dioxide and nitrogen. A common theme among these techniques is the use of a fine (100–10−3 Torr) or high (10−3–10−6 Torr) vacuum to remove air, and the use of an inert gas: preferably argon, but often nitrogen.

Sartorius AG is an international pharmaceutical and laboratory equipment supplier, covering the segments of Bioprocess Solutions and Lab Products & Services.

A microcarrier is a support matrix that allows for the growth of adherent cells in bioreactors. Instead of on a flat surface, cells are cultured on the surface of spherical microcarriers so that each particle carries several hundred cells, and therefore expansion capacity can be multiplied several times over. It provides a straightforward way to scale up culture systems for industrial production of cell or protein-based therapies, or for research purposes.
Plant tissue culture is a collection of techniques used to maintain or grow plant cells, tissues or organs under sterile conditions on a nutrient culture medium of known composition. It is widely used to produce clones of a plant in a method known as micropropagation. Different techniques in plant tissue culture may offer certain advantages over traditional methods of propagation, including:

Cannula transfer or cannulation is a set of air-free techniques used with a Schlenk line, in transferring liquid or solution samples between reaction vessels via cannulae, avoiding atmospheric contamination. While the syringes are not the same as cannulae, the techniques remain relevant.
The Statnamic load test is a type of test for assessing the load-carrying capacity of deep foundations which is faster and less expensive than the static load test. The Statnamic test was conceived in 1985, with the first prototype tests carried out in 1988 through collaboration between Berminghammer Foundation Equipment of Canada and TNO Building Research of the Netherlands. Guidance on rapid load pile testing can be found in: Methods for Axial Compressive Force Pulse (Rapid) Testing of Deep Foundations. Sanken D7383 - 08 Standard Test.
A single-use bioreactor or disposable bioreactor is a bioreactor with a disposable bag instead of a culture vessel. Typically, this refers to a bioreactor in which the lining in contact with the cell culture will be plastic, and this lining is encased within a more permanent structure. Commercial single-use bioreactors have been available since the end of the 1990s and are now made by several well-known producers.

An inoculation needle is a laboratory equipment used in the field of microbiology to transfer and inoculate living microorganisms. It is one of the most commonly implicated biological laboratory tools and can be disposable or re-usable. A standard reusable inoculation needle is made from nichrome or platinum wire affixed to a metallic handle. A disposable inoculation needle is often made from plastic resin. The base of the needle is dulled, resulting in a blunted end.
References
- ↑ ASTM E1287-89(1999) "Standard Practice for Aseptic Sampling of Biological Materials," ASTM International, West Conshohocken, PA, 1999, DOI: 10.1520/E1287-89R99, www.astm.org
- ↑ Stockdale, D. Overview of Aseptic Fill/Finish Manufacturing. Am. Pharm. Rev. 2004.
- ↑ Rosen, Michael. "The Cost of Doing Biotech Business: Midwest cheaper than the coast, but not by much?", WTN News , 2008-09-12. Retrieved on 2009-09-14.