In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or in the terminal position. Terminal alkenes are also known as α-olefins.

In chemistry, an ester is a functional group derived from an acid in which the hydrogen atom (H) of at least one acidic hydroxyl group of that acid is replaced by an organyl group. Analogues derived from oxygen replaced by other chalcogens belong to the ester category as well. According to some authors, organyl derivatives of acidic hydrogen of other acids are esters as well, but not according to the IUPAC.

In organic chemistry, a ketone is an organic compound with the structure R−C(=O)−R', where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group −C(=O)−. The simplest ketone is acetone, with the formula (CH3)2CO. Many ketones are of great importance in biology and industry. Examples include many sugars (ketoses), many steroids, and the solvent acetone.
In organic chemistry, a methyl group is an alkyl derived from methane, containing one carbon atom bonded to three hydrogen atoms, having chemical formula CH3. In formulas, the group is often abbreviated as Me. This hydrocarbon group occurs in many organic compounds. It is a very stable group in most molecules. While the methyl group is usually part of a larger molecule, bonded to the rest of the molecule by a single covalent bond, it can be found on its own in any of three forms: methanide anion, methylium cation or methyl radical. The anion has eight valence electrons, the radical seven and the cation six. All three forms are highly reactive and rarely observed.

The haloalkanes are alkanes containing one or more halogen substituents. They are a subset of the general class of halocarbons, although the distinction is not often made. Haloalkanes are widely used commercially. They are used as flame retardants, fire extinguishants, refrigerants, propellants, solvents, and pharmaceuticals. Subsequent to the widespread use in commerce, many halocarbons have also been shown to be serious pollutants and toxins. For example, the chlorofluorocarbons have been shown to lead to ozone depletion. Methyl bromide is a controversial fumigant. Only haloalkanes that contain chlorine, bromine, and iodine are a threat to the ozone layer, but fluorinated volatile haloalkanes in theory may have activity as greenhouse gases. Methyl iodide, a naturally occurring substance, however, does not have ozone-depleting properties and the United States Environmental Protection Agency has designated the compound a non-ozone layer depleter. For more information, see Halomethane. Haloalkane or alkyl halides are the compounds which have the general formula "RX" where R is an alkyl or substituted alkyl group and X is a halogen.

Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene. Alkylating agents are reagents for effecting alkylation. Alkyl groups can also be removed in a process known as dealkylation. Alkylating agents are often classified according to their nucleophilic or electrophilic character. In oil refining contexts, alkylation refers to a particular alkylation of isobutane with olefins. For upgrading of petroleum, alkylation produces a premium blending stock for gasoline. In medicine, alkylation of DNA is used in chemotherapy to damage the DNA of cancer cells. Alkylation is accomplished with the class of drugs called alkylating antineoplastic agents.

The Appel reaction is an organic reaction that converts an alcohol into an alkyl chloride using triphenylphosphine and carbon tetrachloride. The use of carbon tetrabromide or bromine as a halide source will yield alkyl bromides, whereas using carbon tetraiodide, methyl iodide or iodine gives alkyl iodides. The reaction is credited to and named after Rolf Appel, it had however been described earlier. The use of this reaction is becoming less common, due to carbon tetrachloride being restricted under the Montreal protocol.
In organic chemistry, ozonolysis is an organic reaction where the unsaturated bonds are cleaved with ozone. Multiple carbon–carbon bond are replaced by carbonyl groups, such as aldehydes, ketones, and carboxylic acids. The reaction is predominantly applied to alkenes, but alkynes and azo compounds are also susceptible to cleavage. The outcome of the reaction depends on the type of multiple bond being oxidized and the work-up conditions.
In organic chemistry, the Mannich reaction is a three-component organic reaction that involves the amino alkylation of an acidic proton next to a carbonyl functional group by formaldehyde and a primary or secondary amine or ammonia. The final product is a β-amino-carbonyl compound also known as a Mannich base. Reactions between aldimines and α-methylene carbonyls are also considered Mannich reactions because these imines form between amines and aldehydes. The reaction is named after Carl Mannich.

The Michaelis–Arbuzov reaction is the chemical reaction of a trivalent phosphorus ester with an alkyl halide to form a pentavalent phosphorus species and another alkyl halide. The picture below shows the most common types of substrates undergoing the Arbuzov reaction; phosphite esters (1) react to form phosphonates (2), phosphonites (3) react to form phosphinates (4) and phosphinites (5) react to form phosphine oxides (6).
Dimethyl sulfate (DMS) is a chemical compound with formula (CH3O)2SO2. As the diester of methanol and sulfuric acid, its formula is often written as (CH3)2SO4 or Me2SO4, where CH3 or Me is methyl. Me2SO4 is mainly used as a methylating agent in organic synthesis. Me2SO4 is a colourless oily liquid with a slight onion-like odour. Like all strong alkylating agents, Me2SO4 is toxic. Its use as a laboratory reagent has been superseded to some extent by methyl triflate, CF3SO3CH3, the methyl ester of trifluoromethanesulfonic acid.

The Knorr pyrrole synthesis is a widely used chemical reaction that synthesizes substituted pyrroles (3). The method involves the reaction of an α-amino-ketone (1) and a compound containing an electron-withdrawing group α to a carbonyl group (2).

Camphorsulfonic acid, sometimes abbreviated CSA or 10-CSA is an organosulfur compound. Like typical sulfonic acids, it is a relatively strong acid that is a colorless solid at room temperature and is soluble in water and a wide variety of organic substances.
The Kornblum–DeLaMare rearrangement is a rearrangement reaction in organic chemistry in which a primary or secondary organic peroxide is converted to the corresponding ketone and alcohol under acid or base catalysis. The reaction is relevant as a tool in organic synthesis and is a key step in the biosynthesis of prostaglandins.
Organophosphines are organophosphorus compounds with the formula PRnH3−n, where R is an organic substituent. These compounds can be classified according to the value of n: primary phosphines (n = 1), secondary phosphines (n = 2), tertiary phosphines (n = 3). All adopt pyramidal structures. Organophosphines are generally colorless, lipophilic liquids or solids. The parent of the organophosphines is phosphine (PH3).

Triethyl phosphite is an organophosphorus compound, specifically a phosphite ester, with the formula P(OCH2CH3)3, often abbreviated P(OEt)3. It is a colorless, malodorous liquid. It is used as a ligand in organometallic chemistry and as a reagent in organic synthesis.

Sulfinyl halide have the general formula R−S(O)−X, where X is a halogen. They are intermediate in oxidation level between sulfenyl halides, R−S−X, and sulfonyl halides, R−SO2−X. The best known examples are sulfinyl chlorides, thermolabile, moisture-sensitive compounds, which are useful intermediates for preparation of other sufinyl derivatives such as sulfinamides, sulfinates, sulfoxides, and thiosulfinates. Unlike the sulfur atom in sulfonyl halides and sulfenyl halides, the sulfur atom in sulfinyl halides is chiral, as shown for methanesulfinyl chloride.

Diethyl chlorophosphate is an organophosphorus compound with the formula (C2H5O)2P(O)Cl. As a reagent in organic synthesis, it is used to convert alcohols to the corresponding diethylphosphate esters. It is a colorless liquid with a fruity odor. It is a corrosive, and as a cholinesterase inhibitor, highly toxic through dermal absorption. The molecule is tetrahedral.

Diethyl phosphite is the organophosphorus compound with the formula (C2H5O)2P(O)H. It is a popular reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. Diethyl phosphite is a colorless liquid. The molecule is tetrahedral.

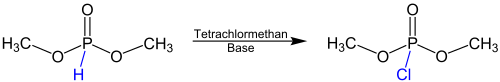
Dimethylphosphite is an organophosphorus compound with the formula (CH3O)2P(O)H, known as dimethyl hydrogen phosphite (DMHP). Dimethylphosphite, is a minor tautomer of the phosphorus(V) derivative. It is a reagent for generating other organophosphorus compounds, exploiting the high reactivity of the P-H bond. The molecule is tetrahedral. It is a colorless liquid. The compounds can be prepared by methanolysis of phosphorus trichloride or by heating diethylphosphite in methanol.