
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− (pyruvic acid), and a hydrogen ion, H+. The free energy released in this process is used to form the high-energy molecules ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide). Glycolysis is a sequence of ten enzyme-catalyzed reactions. Most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful rather than just utilized as steps in the overall reaction. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.

Tequila is a distilled beverage made from the blue agave plant, primarily in the area surrounding the city of Tequila 65 km (40 mi) northwest of Guadalajara, and in the Jaliscan Highlands of the central western Mexican state of Jalisco. Like mezcal, tequila is also made from the agave plant and originates from the same regions of Mexico. The distinction is that tequila is made only from blue agave and they are prepared in different ways. Tequila is commonly served neat in Mexico and as a shot with salt and lime around the world.

In biochemistry, fermentation theory refers to the historical study of models of natural fermentation processes, especially alcoholic and lactic acid fermentation. Notable contributors to the theory include Justus Von Liebig and Louis Pasteur, the latter of whom developed a purely microbial basis for the fermentation process based on his experiments. Pasteur's work on fermentation later led to his development of the germ theory of disease, which put the concept of spontaneous generation to rest. Although the fermentation process had been used extensively throughout history prior to the origin of Pasteur's prevailing theories, the underlying biological and chemical processes were not fully understood. In the contemporary, fermentation is used in the production of various alcoholic beverages, foodstuffs, and medications.

Carbonic maceration is a winemaking technique, often associated with the French wine region of Beaujolais, in which whole grapes are fermented in a carbon dioxide rich environment prior to crushing. Conventional alcoholic fermentation involves crushing the grapes to free the juice and pulp from the skin with yeast serving to convert sugar into ethanol. Carbonic maceration ferments most of the juice while it is still inside the grape, although grapes at the bottom of the vessel are crushed by gravity and undergo conventional fermentation. The resulting wine is fruity with very low tannins. It is ready to drink quickly but lacks the structure for long-term aging. In the most extreme case, such as with Beaujolais nouveau, the period between picking and bottling can be less than six weeks.

Malolactic fermentation is a process in winemaking in which tart-tasting malic acid, naturally present in grape must, is converted to softer-tasting lactic acid. Malolactic fermentation is most often performed as a secondary fermentation shortly after the end of the primary fermentation, but can sometimes run concurrently with it. The process is standard for most red wine production and common for some white grape varieties such as Chardonnay, where it can impart a "buttery" flavor from diacetyl, a byproduct of the reaction.
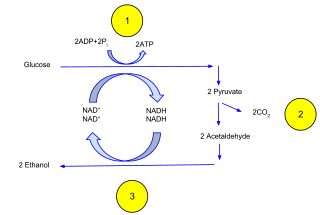
Ethanol fermentation, also called alcoholic fermentation, is a biological process which converts sugars such as glucose, fructose, and sucrose into cellular energy, producing ethanol and carbon dioxide as by-products. Because yeasts perform this conversion in the absence of oxygen, alcoholic fermentation is considered an anaerobic process. It also takes place in some species of fish where it provides energy when oxygen is scarce.
Industrial fermentation is the intentional use of fermentation by microorganisms such as bacteria and fungi as well as eukaryotic cells like CHO cells and insect cells, to make products useful to humans. Fermented products have applications as food as well as in general industry. Some commodity chemicals, such as acetic acid, citric acid, and ethanol are made by fermentation. The rate of fermentation depends on the concentration of microorganisms, cells, cellular components, and enzymes as well as temperature, pH and for aerobic fermentation oxygen. Product recovery frequently involves the concentration of the dilute solution. Nearly all commercially produced enzymes, such as lipase, invertase and rennet, are made by fermentation with genetically modified microbes. In some cases, production of biomass itself is the objective, as in the case of baker's yeast and lactic acid bacteria starter cultures for cheesemaking. In general, fermentations can be divided into four types:

Lees are deposits of dead yeast or residual yeast and other particles that precipitate, or are carried by the action of "fining", to the bottom of a vat of wine after fermentation and aging. The yeast deposits in beer brewing are known as trub. However, yeast deposits from secondary fermentation of both wine and beer are referred to as lees. This material is the source for most commercial tartaric acid, which is used in cooking and in organic chemistry.

Fermentation is a metabolic process that produces chemical changes in organic substrates through the action of enzymes. In biochemistry, it is narrowly defined as the extraction of energy from carbohydrates in the absence of respiration. In the context of food production, it may more broadly refer to any process in which the activity of microorganisms brings about a desirable change to a foodstuff or beverage. The science of fermentation is known as zymology.

In cooking, proofing is a step in the preparation of yeast bread and other baked goods where the dough is allowed to rest and rise a final time before baking. During this rest period, yeast ferment the dough and produce gasses, thereby leavening the dough.

o-Phthalaldehyde or ortho-phthalaldehyde (OPA) is the chemical compound with the formula C6H4(CHO)2. Often abbreviated OPA, the molecule is a dialdehyde, consisting of two formyl (CHO) groups attached to adjacent carbon centres on a benzene ring. This pale yellow solid is a building block in the synthesis of heterocyclic compounds and a reagent in the analysis of amino acids. OPA dissolves in water solution at pH < 11.5. Its solutions degrade upon UV illumination and exposure to air.

The process of fermentation in winemaking turns grape juice into an alcoholic beverage. During fermentation, yeasts transform sugars present in the juice into ethanol and carbon dioxide. In winemaking, the temperature and speed of fermentation are important considerations as well as the levels of oxygen present in the must at the start of the fermentation. The risk of stuck fermentation and the development of several wine faults can also occur during this stage, which can last anywhere from 5 to 14 days for primary fermentation and potentially another 5 to 10 days for a secondary fermentation. Fermentation may be done in stainless steel tanks, which is common with many white wines like Riesling, in an open wooden vat, inside a wine barrel and inside the wine bottle itself as in the production of many sparkling wines.

The aromas of wine are more diverse than its flavors. The human tongue is limited to the primary tastes perceived by taste receptors on the tongue – sourness, bitterness, saltiness, sweetness and savoriness. The wide array of fruit, earthy, leathery, floral, herbal, mineral, and woodsy flavor present in wine are derived from aroma notes sensed by the olfactory bulb. In wine tasting, wine is sometimes smelled before being drunk in order to identify some components of the wine that may be present. Different terms are used to describe what is being smelled. The most basic term is aroma which generally refers to a "pleasant" smell as opposed to odor which refers to an unpleasant smell or possible wine fault. The term aroma may be further distinguished from bouquet which generally refers to the smells that arise from the chemical reactions of fermentation and aging of the wine.
This glossary of winemaking terms lists some of terms and definitions involved in making wine, fruit wine, and mead.

Autolysis in winemaking relates to the complex chemical reactions that take place when a wine spends time in contact with the lees, or dead yeast cells, after fermentation. While for some wines - and all beers - autolysis is undesirable, it is a vital component in shaping the flavors and mouth feel associated with premium Champagne production. The practice of leaving a wine to age on its lees has a long history in winemaking dating back to Roman winemaking. The chemical process and details of autolysis were not originally understood scientifically, but the positive effects such as a creamy mouthfeel, breadlike and floral aromas, and reduced astringency were noticed early in the history of wine.
Liebig–Pasteur dispute is the dispute between Justus von Liebig and Louis Pasteur on the processes and causes of fermentation.

The role of yeast in winemaking is the most important element that distinguishes wine from grape juice. In the absence of oxygen, yeast converts the sugars of wine grapes into alcohol and carbon dioxide through the process of fermentation. The more sugars in the grapes, the higher the potential alcohol level of the wine if the yeast are allowed to carry out fermentation to dryness. Sometimes winemakers will stop fermentation early in order to leave some residual sugars and sweetness in the wine such as with dessert wines. This can be achieved by dropping fermentation temperatures to the point where the yeast are inactive, sterile filtering the wine to remove the yeast or fortification with brandy or neutral spirits to kill off the yeast cells. If fermentation is unintentionally stopped, such as when the yeasts become exhausted of available nutrients and the wine has not yet reached dryness, this is considered a stuck fermentation.

Yeast assimilable nitrogen or YAN is the combination of Free Amino Nitrogen (FAN), ammonia (NH3) and ammonium (NH4+) that is available for the wine yeast Saccharomyces cerevisiae to use during fermentation. Outside of the fermentable sugars glucose and fructose, nitrogen is the most important nutrient needed to carry out a successful fermentation that doesn't end prior to the intended point of dryness or sees the development of off-odors and related wine faults. To this extent winemakers will often supplement the available YAN resources with nitrogen additives such as diammonium phosphate (DAP).

The chemical compounds in beer give it a distinctive taste, smell and appearance. The majority of compounds in beer come from the metabolic activities of plants and yeast and so are covered by the fields of biochemistry and organic chemistry. The main exception is that beer contains over 90% water and the mineral ions in the water (hardness) can have a significant effect upon the taste.

A beer fault or defect is caused by the chemical change of organic matter in beer due to the wrong production process and storage mode and leads to beer deterioration. The chemicals that cause flavour defects in beer have been produced in beer. For example, the aldehydes such as dactyl organic acids, lipids and sulfur compounds in beer influence the taste of beer. When the concentration of one or more elements exceeds the standard threshold, the flavour characteristics of beer will change and form the flavour defect of beer. In particular, fermentation byproducts, even small fluctuations of just over 1% above the threshold can have an impact on the flavor of the beer.
















