
Scoliosis is a condition in which a person's spine has an irregular curve in the coronal plane. The curve is usually S- or C-shaped over three dimensions. In some, the degree of curve is stable, while in others, it increases over time. Mild scoliosis does not typically cause problems, but more severe cases can affect breathing and movement. Pain is usually present in adults, and can worsen with age. As the condition progresses, it may alter a person's life, and hence can also be considered a disability. It can be compared to kyphosis and lordosis, other abnormal curvatures of the spine which are in the sagittal plane (front-back) rather than the coronal (left-right).

Lumbar puncture (LP), also known as a spinal tap, is a medical procedure in which a needle is inserted into the spinal canal, most commonly to collect cerebrospinal fluid (CSF) for diagnostic testing. The main reason for a lumbar puncture is to help diagnose diseases of the central nervous system, including the brain and spine. Examples of these conditions include meningitis and subarachnoid hemorrhage. It may also be used therapeutically in some conditions. Increased intracranial pressure is a contraindication, due to risk of brain matter being compressed and pushed toward the spine. Sometimes, lumbar puncture cannot be performed safely. It is regarded as a safe procedure, but post-dural-puncture headache is a common side effect if a small atraumatic needle is not used.

Kyphosis is an abnormally excessive convex curvature of the spine as it occurs in the thoracic and sacral regions. Abnormal inward concave lordotic curving of the cervical and lumbar regions of the spine is called lordosis.

The Harrington rod is a stainless steel surgical device. Historically, this rod was implanted along the spinal column to treat, among other conditions, a lateral or coronal-plane curvature of the spine, or scoliosis. Up to one million people had Harrington rods implanted for scoliosis between the early 1960s and the late 1990s.

Personalized medicine, also referred to as precision medicine, is a medical model that separates people into different groups—with medical decisions, practices, interventions and/or products being tailored to the individual patient based on their predicted response or risk of disease. The terms personalized medicine, precision medicine, stratified medicine and P4 medicine are used interchangeably to describe this concept, though some authors and organizations differentiate between these expressions based on particular nuances. P4 is short for "predictive, preventive, personalized and participatory".

A back brace is a device designed to limit the motion of the spine in cases of bone fracture or in post-operative spinal fusiona, as well as a preventative measure against some progressive conditions or to correct patient posture.
In medicine, a biomarker is a measurable indicator of the severity or presence of some disease state. It may be defined as a "cellular, biochemical or molecular alteration in cells, tissues or fluids that can be measured and evaluated to indicate normal biological processes, pathogenic processes, or pharmacological responses to a therapeutic intervention." More generally a biomarker is anything that can be used as an indicator of a particular disease state or some other physiological state of an organism. According to the WHO, the indicator may be chemical, physical, or biological in nature - and the measurement may be functional, physiological, biochemical, cellular, or molecular.

Kyphoscoliosis describes an abnormal curvature of the spine in both the coronal and sagittal planes. It is a combination of kyphosis and scoliosis. This musculoskeletal disorder often leads to other issues in patients, such as under-ventilation of lungs, pulmonary hypertension, difficulty in performing day-to-day activities, and psychological issues emanating from anxiety about acceptance among peers, especially in young patients. It can also be seen in syringomyelia, Friedreich's ataxia, spina bifida, kyphoscoliotic Ehlers–Danlos syndrome (kEDS), and Duchenne muscular dystrophy due to asymmetric weakening of the paraspinal muscles.
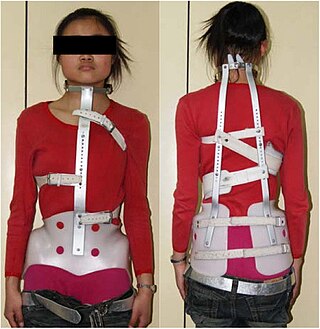
The Milwaukee brace, also known as a cervico-thoraco-lumbo-sacral orthosis or CTLSO, is a back brace most often used in the treatment of spinal curvatures in children but also, more rarely, in adults to prevent collapse of the spine and associated pain and deformity. It is a full-torso brace that extends from the pelvis to the base of the skull. It was originally designed by Blount and Schmidt in 1946 for postoperative care when surgery required long periods of immobilization.
The Boston brace, a type of thoraco-lumbo-sacral-orthosis (TLSO), is a back brace used primarily for the treatment of idiopathic scoliosis in children. It was developed in 1972 by M.E "Bill" Miller and John Hall at the Boston Children's Hospital in Boston, Massachusetts.

Spinal disease refers to a condition impairing the backbone. These include various diseases of the back or spine ("dorso-"), such as kyphosis. Dorsalgia refers to back pain. Some other spinal diseases include spinal muscular atrophy, ankylosing spondylitis, scoliosis, lumbar spinal stenosis, spina bifida, spinal tumors, osteoporosis and cauda equina syndrome.

The Scoliosis Research Society (SRS) is a non-profit, professional, international organization made up of physicians and allied health personnel, whose purpose is to "care for those with spinal deformity throughout life by patient care, education, research and patient advocacy." It was founded in 1966 with 37 members, and now has grown to include over 1300 spinal deformity surgeons and allied health personnel in 41 countries, with a primary focus on providing continuing medical education for health care professionals, and funding/support for research in spinal deformities. Among the founding members were Dr. Paul Randall Harrington, inventor of the Harrington rod treatment for scoliosis, and Dr. David B. Levine, spine surgeon at Hospital for Special Surgery. Harrington later served as President of the SRS from 1972 to 1973, and Levine was President of the Society from 1978 to 1979. Current membership primarily includes spinal deformity surgeons, as well as some researchers, physician assistants, and orthotists who are involved in research and treatment of spinal deformities. Strict membership criteria ensure that the individual SRS Fellows are dedicated to the highest standards of care for adult and pediatric spinal deformities, utilizing both non-operative and operative techniques.

The neuromechanics of idiopathic scoliosis is about the changes in the bones, muscles and joints in cases of spinal deformity consisting of a lateral curvature scoliosis and a rotation of the vertebrae within the curve, that is not explained by either congenital vertebral abnormalities, or neuromuscular disorders such as muscular dystrophy. The idiopathic scoliosis accounts for 80–90% of scoliosis cases. Its pathogenesis is unknown. However, changes in the vestibular system, a lateral shift of the hand representation and abnormal variability of erector spinae motor map location in the motor cortex may be involved in this disease. A short spinal cord and associated nerve tensions has been proposed as a cause and model for idiopathic scoliosis. Besides idiopathic scoliosis being more frequent in certain families, it is suspected to be transmitted via autosomal dominant inheritance. Estrogens could also play a crucial part in the progression of idiopathic scoliosis through their roles in bone formation, growth, maturation and turnover. Finally, collagen, intervertebral disc and muscle abnormalities have been suggested as the cause in idiopathic scoliosis, although these are perhaps results rather than causes.
Lateral electrical surface stimulation is a neuromuscular stimulation treatment for idiopathic scoliosis. It is also known as the LESS treatment, and was invented by Dr. Jens Axelgaard in 1976. It is a non-invasive scoliosis treatment that utilizes electrical muscle stimulation, which is also known as neurostimulation or neuromuscular stimulation.
The management of scoliosis is complex and is determined primarily by the type of scoliosis encountered: syndromic, congenital, neuromuscular, or idiopathic. Treatment options for idiopathic scoliosis are determined in part by the severity of the curvature and skeletal maturity, which together help predict the likelihood of progression. Non-surgical treatment should be pro-active with intervention performed early as "Best results were obtained in 10-25 degrees scoliosis which is a good indication to start therapy before more structural changes within the spine establish." Treatment options have historically been categorized under the following types:
- Observation
- Bracing
- Specialized physical therapy
- Surgery
Anterior vertebral body tethering (AVBT) is a relatively new surgery for the treatment of scoliosis in pediatric patients. Left untreated, severe scoliosis can worsen and eventually affect a person's lungs and heart.
The Providence brace is a nighttime spinal orthosis for the treatment of adolescent idiopathic scoliosis (AIS). The brace is used to curb the natural progression of scoliosis and prevent further curvature of the AIS patient's spine. The Providence brace was developed by Charles d'Amato and Barry McCoy, and is manufactured by Spinal Technology, Inc.

Adolescent idiopathic scoliosis (AIS) is a disorder in which the spine starts abnormally curving sideways (scoliosis) between the ages of 10–18 years old. Generally, AIS occurs during the growth spurt associated with adolescence. In some teens, the curvature is progressive, meaning that it gets worse over time, however, AIS more commonly manifests only as a mild curvature.
Pierre Stagnara was a French spinal surgeon. He has been described as a "pioneer" in the study of spinal deformities, "one of the greatest figures of French spinal surgery." Stagnara was born in January 16, 1917 in Loriol-sur-Drôme, France. He studied medicine in the city of Lyon. During World War II he was drafted into the French army. After the war, he worked in a variety of hospitals throughout Lyon. In 1959 he became the Chief of the Centre des Massues and served in this position until his retirement in 1982. Whilst working at the institution he pioneered many orthopedic techniques. Including the non-surgical management of scoliosis.
Rigid spine syndrome, also known as congenital muscular dystrophy with rigidity of the spine (CMARS), is a rare and often debilitating neuromuscular disorder. It is characterized by progressive muscle stiffness and rigidity, particularly in the spine, which can severely limit mobility and impact quality of life. This condition is typically present from birth or early childhood and tends to worsen over time.









