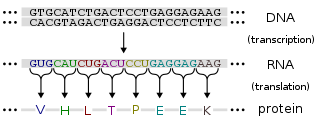
Protein production is the biotechnological process of generating a specific protein. It is typically achieved by the manipulation of gene expression in an organism such that it expresses large amounts of a recombinant gene. This includes the transcription of the recombinant DNA to messenger RNA (mRNA), the translation of mRNA into polypeptide chains, which are ultimately folded into functional proteins and may be targeted to specific subcellular or extracellular locations.

Chinese hamster ovary (CHO) cells are a family of immortalized cell lines derived from epithelial cells of the ovary of the Chinese hamster, often used in biological and medical research and commercially in the production of recombinant therapeutic proteins. They have found wide use in studies of genetics, toxicity screening, nutrition and gene expression, and particularly since the 1980s to express recombinant proteins. CHO cells are the most commonly used mammalian hosts for industrial production of recombinant protein therapeutics.
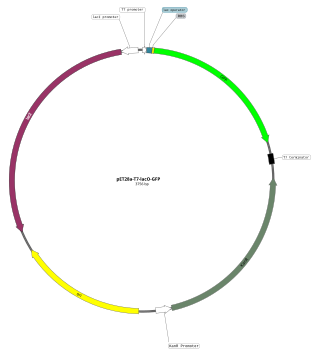
An expression vector, otherwise known as an expression construct, is usually a plasmid or virus designed for gene expression in cells. The vector is used to introduce a specific gene into a target cell, and can commandeer the cell's mechanism for protein synthesis to produce the protein encoded by the gene. Expression vectors are the basic tools in biotechnology for the production of proteins.
Transfection is the process of deliberately introducing naked or purified nucleic acids into eukaryotic cells. It may also refer to other methods and cell types, although other terms are often preferred: "transformation" is typically used to describe non-viral DNA transfer in bacteria and non-animal eukaryotic cells, including plant cells. In animal cells, transfection is the preferred term as transformation is also used to refer to progression to a cancerous state (carcinogenesis) in these cells. Transduction is often used to describe virus-mediated gene transfer into eukaryotic cells.

Adeno-associated viruses (AAV) are small viruses that infect humans and some other primate species. They belong to the genus Dependoparvovirus, which in turn belongs to the family Parvoviridae. They are small replication-defective, nonenveloped viruses and have linear single-stranded DNA (ssDNA) genome of approximately 4.8 kilobases (kb).

Baculoviridae is a family of viruses. Arthropods, among the most studied being Lepidoptera, Hymenoptera and Diptera, serve as natural hosts. Currently, 85 species are placed in this family, assigned to four genera.

The C3a receptor also known as complement component 3a receptor 1 (C3AR1) is a G protein-coupled receptor protein involved in the complement system.
Neurturin (NRTN) is a protein that is encoded in humans by the NRTN gene. Neurturin belongs to the glial cell line-derived neurotrophic factor (GDNF) family of neurotrophic factors, which regulate the survival and function of neurons. Neurturin’s role as a growth factor places it in the transforming growth factor beta (TGF-beta) subfamily along with its homologs persephin, artemin, and GDNF. It shares a 42% similarity in amino acid sequence with mature GDNF. It is also considered a trophic factor and critical in the development and growth of neurons in the brain. Neurotrophic factors like neurturin have been tested in several clinical trial settings for the potential treatment of neurodegenerative diseases, specifically Parkinson's disease.
Rhodopsin kinase is a serine/threonine-specific protein kinase involved in phototransduction. This enzyme catalyses the following chemical reaction:

Baculoviral IAP repeat-containing protein3 is a protein that in humans is encoded by the BIRC3 gene.

Olfactory receptor 2K2 is a protein that in humans is encoded by the OR2K2 gene.

Olfactory receptor 7A5 is a protein that in humans is encoded by the OR7A5 gene.

Olfactory receptor 51E1 is a protein that in humans is encoded by the OR51E1 gene.

Guanine nucleotide-binding protein subunit alpha-11 is a protein that in humans is encoded by the GNA11 gene. Together with GNAQ, it functions as a Gq alpha subunit.
A subunit vaccine is a vaccine that contains purified parts of the pathogen that are antigenic, or necessary to elicit a protective immune response. Subunit vaccine can be made from dissembled viral particles in cell culture or recombinant DNA expression, in which case it is a recombinant subunit vaccine.
Heterologous expression refers to the expression of a gene or part of a gene in a host organism that does not naturally have the gene or gene fragment in question. Insertion of the gene in the heterologous host is performed by recombinant DNA technology. The purpose of heterologous expression is often to determine the effects of mutations and differential interactions on protein function. It provides an easy path to efficiently express and experiment with combinations of genes and mutants that do not naturally occur.
The PiggyBac (PB) transposon system employs a genetically engineered transposase enzyme to insert a gene into a cell's genome. It is built upon the natural PiggyBac (PB) transposable element (transposon), enabling the back and forth movement of genes between chromosomes and genetic vectors such as plasmids through a "cut and paste" mechanism. During transposition, the PB transposase recognizes transposon-specific inverted terminal repeat sequences (ITRs) located on both ends of the transposon vector and efficiently moves the contents from the original sites and integrates them into TTAA chromosomal sites. The powerful activity of the PiggyBac transposon system enables genes of interest between the two ITRs in the PB vector to be easily mobilized into target genomes. The TTAA-specific transposon piggyBac is rapidly becoming a highly useful transposon for genetic engineering of a wide variety of species, particularly insects. They were discovered in 1989 by Malcolm Fraser at the University of Notre Dame.

The Early 35 kDa protein, or P35 in short, is a baculoviral protein that inhibits apoptosis in the cells infected by the virus. Although baculoviruses infect only invertebrates in nature, ectopic expression of P35 in vertebrate animals and cells also results in inhibition of apoptosis, thus indicating a universal mechanism. P35 has been shown to be a caspase inhibitor with a very wide spectrum of activity both in regard to inhibited caspase types and to species in which the mechanism is conserved.

Olfactory receptor family 1 subfamily E member 3 (gene/pseudogene) is a protein that in humans is encoded by the OR1E3 gene.
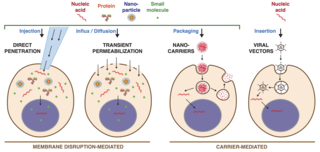
Intracellular delivery is the process of introducing external materials into living cells. Materials that are delivered into cells include nucleic acids, proteins, peptides, impermeable small molecules, synthetic nanomaterials, organelles, and micron-scale tracers, devices and objects. Such molecules and materials can be used to investigate cellular behavior, engineer cell operations or correct a pathological function.













