Related Research Articles
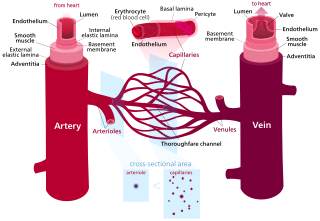
Blood vessels are the structures of the circulatory system that transport blood throughout the human body. These vessels transport blood cells, nutrients, and oxygen to the tissues of the body. They also take waste and carbon dioxide away from the tissues. Blood vessels are needed to sustain life, because all of the body's tissues rely on their functionality.
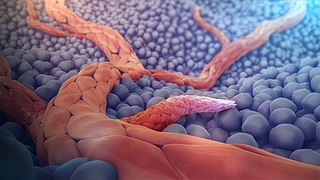
Angiogenesis is the physiological process through which new blood vessels form from pre-existing vessels, formed in the earlier stage of vasculogenesis. Angiogenesis continues the growth of the vasculature mainly by processes of sprouting and splitting, but processes such as coalescent angiogenesis, vessel elongation and vessel cooption also play a role. Vasculogenesis is the embryonic formation of endothelial cells from mesoderm cell precursors, and from neovascularization, although discussions are not always precise. The first vessels in the developing embryo form through vasculogenesis, after which angiogenesis is responsible for most, if not all, blood vessel growth during development and in disease.

A monoclonal antibody is an antibody produced from a cell lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell.

The microcirculation is the circulation of the blood in the smallest blood vessels, the microvessels of the microvasculature present within organ tissues. The microvessels include terminal arterioles, metarterioles, capillaries, and venules. Arterioles carry oxygenated blood to the capillaries, and blood flows out of the capillaries through venules into veins.
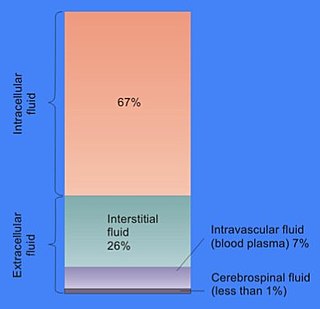
In cell biology, extracellular fluid (ECF) denotes all body fluid outside the cells of any multicellular organism. Total body water in healthy adults is about 50–60% of total body weight; women and the obese typically have a lower percentage than lean men. Extracellular fluid makes up about one-third of body fluid, the remaining two-thirds is intracellular fluid within cells. The main component of the extracellular fluid is the interstitial fluid that surrounds cells.

Chinese hamster ovary (CHO) cells are a family of immortalized cell lines derived from epithelial cells of the ovary of the Chinese hamster, often used in biological and medical research and commercially in the production of recombinant therapeutic proteins. They have found wide use in studies of genetics, toxicity screening, nutrition and gene expression, and particularly since the 1980s to express recombinant proteins. CHO cells are the most commonly used mammalian hosts for industrial production of recombinant protein therapeutics.

The dermis or corium is a layer of skin between the epidermis and subcutaneous tissues, that primarily consists of dense irregular connective tissue and cushions the body from stress and strain. It is divided into two layers, the superficial area adjacent to the epidermis called the papillary region and a deep thicker area known as the reticular dermis. The dermis is tightly connected to the epidermis through a basement membrane. Structural components of the dermis are collagen, elastic fibers, and extrafibrillar matrix. It also contains mechanoreceptors that provide the sense of touch and thermoreceptors that provide the sense of heat. In addition, hair follicles, sweat glands, sebaceous glands, apocrine glands, lymphatic vessels, nerves and blood vessels are present in the dermis. Those blood vessels provide nourishment and waste removal for both dermal and epidermal cells.

A bioreactor refers to any manufactured device or system that supports a biologically active environment. In one case, a bioreactor is a vessel in which a chemical process is carried out which involves organisms or biochemically active substances derived from such organisms. This process can either be aerobic or anaerobic. These bioreactors are commonly cylindrical, ranging in size from litres to cubic metres, and are often made of stainless steel. It may also refer to a device or system designed to grow cells or tissues in the context of cell culture. These devices are being developed for use in tissue engineering or biochemical/bioprocess engineering.

Hybridoma technology is a method for producing large numbers of identical antibodies, also called monoclonal antibodies. This process starts by injecting a mouse with an antigen that provokes an immune response. A type of white blood cell, the B cell, produces antibodies that bind to the injected antigen. These antibody producing B-cells are then harvested from the mouse and, in turn, fused with immortal myeloma cancer cells, to produce a hybrid cell line called a hybridoma, which has both the antibody-producing ability of the B-cell and the longevity and reproductivity of the myeloma.

Loose connective tissue, also known as areolar tissue, is a cellular connective tissue with thin and relatively sparse collagen fibers. They have a semi-fluid matrix with lesser proportions of fibers. Its ground substance occupies more volume than the fibers do. It has a viscous to gel-like consistency and plays an important role in the diffusion of oxygen and nutrients from the capillaries that course through this connective tissue as well as in the diffusion of carbon dioxide and metabolic wastes back to the vessels. Moreover, loose connective tissue is primarily located beneath the epithelia that cover the body surfaces and line the internal surfaces of the body. It is also associated with the epithelium of glands and surrounds the smallest blood vessels. This tissue is thus the initial site where pathogenic agents, such as bacteria that have breached an epithelial surface, are challenged and destroyed by cells of the immune system.
A biopharmaceutical, also known as a biological medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, whole blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living medicines used in cell therapy. Biologics can be composed of sugars, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. They are isolated from living sources—human, animal, plant, fungal, or microbial. They can be used in both human and animal medicine.
Industrial fermentation is the intentional use of fermentation in manufacturing processes. In addition to the mass production of fermented foods and drinks, industrial fermentation has widespread applications in chemical industry. Commodity chemicals, such as acetic acid, citric acid, and ethanol are made by fermentation. Moreover, nearly all commercially produced industrial enzymes, such as lipase, invertase and rennet, are made by fermentation with genetically modified microbes. In some cases, production of biomass itself is the objective, as is the case for single-cell proteins, baker's yeast, and starter cultures for lactic acid bacteria used in cheesemaking.
Fed-batch culture is, in the broadest sense, defined as an operational technique in biotechnological processes where one or more nutrients (substrates) are fed (supplied) to the bioreactor during cultivation and in which the product(s) remain in the bioreactor until the end of the run. An alternative description of the method is that of a culture in which "a base medium supports initial cell culture and a feed medium is added to prevent nutrient depletion". It is also a type of semi-batch culture. In some cases, all the nutrients are fed into the bioreactor. The advantage of the fed-batch culture is that one can control concentration of fed-substrate in the culture liquid at arbitrarily desired levels.

A microcarrier is a support matrix that allows for the growth of adherent cells in bioreactors. Instead of on a flat surface, cells are cultured on the surface of spherical microcarriers so that each particle carries several hundred cells, and therefore expansion capacity can be multiplied several times over. It provides a straightforward way to scale up culture systems for industrial production of cell or protein-based therapies, or for research purposes.
Membrane bioreactors are combinations of membrane processes like microfiltration or ultrafiltration with a biological wastewater treatment process, the activated sludge process. These technologies are now widely used for municipal and industrial wastewater treatment. The two basic membrane bioreactor configurations are the submerged membrane bioreactor and the side stream membrane bioreactor. In the submerged configuration, the membrane is located inside the biological reactor and submerged in the wastewater, while in a side stream membrane bioreactor, the membrane is located outside the reactor as an additional step after biological treatment.

Antibody Solutions is a privately held American contract research organization headquartered in Santa Clara, California. It provides research and discovery services and fit-for-purpose antibodies to biopharmaceutical and diagnostic companies and academic researchers worldwide. The company’s services include monoclonal and polyclonal antibody and antigen development, molecular modeling, antibody sequencing and engineering, bioreactor technology, pharmacokinetic studies, antibody epitope binning, peptide synthesis, immunoassay development, ligand-binding assay analysis, and support for CAR-T research.
A rabbit hybridoma is a hybrid cell line formed by the fusion of an antibody producing rabbit B cell with a cancerous B-cell (myeloma).
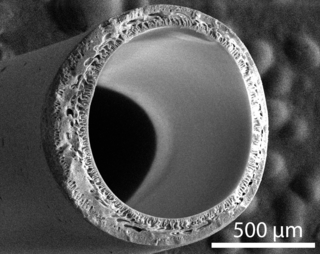
Hollow fiber membranes (HFMs) are a class of artificial membranes containing a semi-permeable barrier in the form of a hollow fiber. Originally developed in the 1960s for reverse osmosis applications, hollow fiber membranes have since become prevalent in water treatment, desalination, cell culture, medicine, and tissue engineering. Most commercial hollow fiber membranes are packed into cartridges which can be used for a variety of liquid and gaseous separations.
Recombinant antibodies are antibody fragments produced by using recombinant antibody coding genes. They mostly consist of a heavy and light chain of the variable region of immunoglobulin. Recombinant antibodies have many advantages in both medical and research applications, which make them a popular subject of exploration and new production against specific targets. The most commonly used form is the single chain variable fragment (scFv), which has shown the most promising traits exploitable in human medicine and research. In contrast to monoclonal antibodies produced by hybridoma technology, which may lose the capacity to produce the desired antibody over time or the antibody may undergo unwanted changes, which affect its functionality, recombinant antibodies produced in phage display maintain high standard of specificity and low immunogenicity.

A cell suspension or suspension culture is a type of cell culture in which single cells or small aggregates of cells are allowed to function and multiply in an agitated growth medium, thus forming a suspension. Suspension culture is one of the two classical types of cell culture, the other being adherent culture. The history of suspension cell culture closely aligns with the history of cell culture overall, but differs in maintenance methods and commercial applications. The cells themselves can either be derived from homogenized tissue or from heterogenous cell solutions. Suspension cell culture is commonly used to culture nonadhesive cell lines like hematopoietic cells, plant cells, and insect cells. While some cell lines are cultured in suspension, the majority of commercially available mammalian cell lines are adherent. Suspension cell cultures must be agitated to maintain cells in suspension, and may require specialized equipment and flasks. These cultures need to be maintained with nutrient containing media and cultured in a specific cell density range to avoid cell death.
References
- ↑ Hirschel M, Gangemi JD, McSharry J., Myers C. Novel Uses for Hollow Fiber Bioreactors Genetic Engineering News Jun 15, 2011 (Vol. 31, No. 12).
- ↑ Knazek RA, Gullino PM, Kohler PO, Dedrick RL. Cell culture on artificial capillaries: an approach to tissue growth in vitro. Science. 1972 Oct 6;178(4056):65-6.
- ↑ Cell culture on semi-permeable tubular membranes U.S. Patent US 3821087 A
- ↑ Ahern, H. Hollow Fiber Bioreactor Systems Increase Cell Culture Yield The Scientist Magazine (1990)
- ↑ Extra-capillary fluid cycling system and method for a cell culture. U.S. Patent US 20130058907 A1
- ↑ Kohler, G., and C. Milstein. 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256:495.
- ↑ Gramer, MJ. Britton TL. Selection and Isolation of Cells for Optimal Growth in Hollow Fiber Bioreactors Hybridoma 2000. 19(5):407-412.
- ↑ De Napoli, Ilaria E.; Zanetti, Elisabetta M.; Fragomeni, Gionata; Giuzio, Ermenegildo; Audenino, Alberto L.; Catapano, Gerardo (2014). "Transport modeling of convection-enhanced hollow fiber membrane bioreactors for therapeutic applications". Journal of Membrane Science. 471: 347–361. doi:10.1016/j.memsci.2014.08.026.
- ↑ Tapia, F. et al. Production of high-titer human influenza A virus with adherent and suspension MDCK cells cultured in a single-use hollow fiber bioreactor Vaccine 32 (2014): 1003-1011.