Related Research Articles

Manihot esculenta, commonly called cassava, manioc, or yuca, is a woody shrub of the spurge family, Euphorbiaceae, native to South America, from Brazil, Paraguay and parts of the Andes. Although a perennial plant, cassava is extensively cultivated in tropical and subtropical regions as an annual crop for its edible starchy tuberous root. Cassava is predominantly consumed in boiled form, but substantial quantities are processed to extract cassava starch, called tapioca, which is used for food, animal feed, and industrial purposes. The Brazilian farofa, and the related garri of West Africa, is an edible coarse flour obtained by grating cassava roots, pressing moisture off the obtained grated pulp, and finally drying it.

Erwinia is a genus of Enterobacterales bacteria containing mostly plant pathogenic species which was named for the famous plant pathologist, Erwin Frink Smith. It contains Gram-negative bacteria related to Escherichia coli, Shigella, Salmonella, and Yersinia. They are primarily rod-shaped bacteria.

A leaf spot is a limited, discoloured, diseased area of a leaf that is caused by fungal, bacterial or viral plant diseases, or by injuries from nematodes, insects, environmental factors, toxicity or herbicides. These discoloured spots or lesions often have a centre of necrosis. Symptoms can overlap across causal agents, however differing signs and symptoms of certain pathogens can lead to the diagnosis of the type of leaf spot disease. Prolonged wet and humid conditions promote leaf spot disease and most pathogens are spread by wind, splashing rain or irrigation that carry the disease to other leaves.

Citrus canker is a disease affecting Citrus species caused by the bacterium Xanthomonas. Infection causes lesions on the leaves, stems, and fruit of citrus trees, including lime, oranges, and grapefruit. While not harmful to humans, canker significantly affects the vitality of citrus trees, causing leaves and fruit to drop prematurely; a fruit infected with canker is safe to eat, but too unsightly to be sold. Citrus canker is mainly a leaf-spotting and rind-blemishing disease, but when conditions are highly favorable, it can cause defoliation, shoot dieback, and fruit drop.
Bacterial blight is a disease of barley caused by the bacterial pathogen Xanthomonas campestris pv. translucens. It has been known as a disease since the late 19th century. It has a worldwide distribution.

Xanthomonas campestris is a gram-negative, obligate aerobic bacterium that is a member of the Xanthomonas genus, which is a group of bacteria that are commonly known for their association with plant disease. This species includes Xanthomonas campestris pv. campestris, the cause of black rot in brassicas, one of the most important diseases of brassicas worldwide.

Ralstonia solanacearum is an aerobic non-spore-forming, Gram-negative, plant pathogenic bacterium. R. solanacearum is soil-borne and motile with a polar flagellar tuft. It colonises the xylem, causing bacterial wilt in a very wide range of potential host plants. It is known as Granville wilt when it occurs in tobacco. Bacterial wilts of tomato, pepper, eggplant, and Irish potato caused by R. solanacearum were among the first diseases that Erwin Frink Smith proved to be caused by a bacterial pathogen. Because of its devastating lethality, R. solanacearum is now one of the more intensively studied phytopathogenic bacteria, and bacterial wilt of tomato is a model system for investigating mechanisms of pathogenesis. Ralstonia was until recently classified as Pseudomonas, with similarity in most aspects, except that it does not produce fluorescent pigment like Pseudomonas. The genomes from different strains vary from 5.5 Mb up to 6 Mb, roughly being 3.5 Mb of a chromosome and 2 Mb of a megaplasmid. While the strain GMI1000 was one of the first phytopathogenic bacteria to have its genome completed, the strain UY031 was the first R. solanacearum to have its methylome reported. Within the R. solanacearum species complex, the four major monophyletic clusters of strains are termed phylotypes, that are geographically distinct: phylotypes I-IV are found in Asia, the Americas, Africa, and Oceania, respectively.

Xanthomonas is a genus of bacteria, many of which cause plant diseases. There are at least 27 plant associated Xanthomonas spp., that all together infect at least 400 plant species. Different species typically have specific host and/or tissue range and colonization strategies.
Xanthomonas arboricola is a species of bacteria. This phytopathogenic bacterium can cause disease in trees like Prunus, hazelnut and walnut.
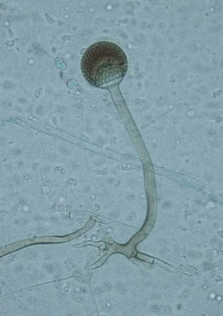
Rhizopus microsporus is a fungal plant pathogen infecting maize, sunflower, and rice.

Plant disease resistance protects plants from pathogens in two ways: by pre-formed structures and chemicals, and by infection-induced responses of the immune system. Relative to a susceptible plant, disease resistance is the reduction of pathogen growth on or in the plant, while the term disease tolerance describes plants that exhibit little disease damage despite substantial pathogen levels. Disease outcome is determined by the three-way interaction of the pathogen, the plant, and the environmental conditions.
Black rot, caused by the bacterium Xanthomonas campestris pv. campestris (Xcc), is considered the most important and most destructive disease of crucifers, infecting all cultivated varieties of brassicas worldwide. This disease was first described by botanist and entomologist Harrison Garman in Lexington, Kentucky, US in 1889. Since then, it has been found in nearly every country in which vegetable brassicas are commercially cultivated.

Banana Xanthomonas Wilt (BXW), or banana bacterial wilt (BBW) or enset wilt is a bacterial disease caused by Xanthomonas vasicola pv. musacearum. After being originally identified on a close relative of banana, Ensete ventricosum, in Ethiopia in the 1960s, BXW emanated in Uganda in 2001 affecting all types of banana cultivars. Since then BXW has been diagnosed in Central and East Africa including banana growing regions of: Rwanda, Democratic Republic of the Congo, Tanzania, Kenya, Burundi, and Uganda.

Xanthomonas campestris pv. vesicatoria is a bacterium that causes bacterial leaf spot (BLS) on peppers and tomatoes. It is a gram-negative and rod-shaped. It causes symptoms throughout the above-ground portion of the plant including leaf spots, fruit spots and stem cankers. Since this bacterium cannot live in soil for more than a few weeks and survives as inoculum on plant debris, removal of dead plant material and chemical applications to living plants are considered effective control mechanisms.
Bacterial blight of cotton is a disease affecting the cotton plant resulting from infection by Xanthomonas axonopodis pathovar malvacearum (Xcm) a Gram negative, motile rod-shaped, non spore-forming bacterium with a single polar flagellum
Bacterial wilt of turfgrass is the only known bacterial disease of turf. The causal agent is the Gram negative bacterium Xanthomonas translucens pv. graminis. The first case of bacterial wilt of turf was reported in a cultivar of creeping bentgrass known as Toronto or C-15, which is found throughout the midwestern United States. Until the causal agent was identified in 1984, the disease was referred to simply as C-15 decline. This disease is almost exclusively found on putting greens at golf courses where extensive mowing creates wounds in the grass which the pathogen uses in order to enter the host and cause disease.

Xanthomonas oryzae pv. oryzae is a bacterial pathovar that causes a serious blight of rice, other grasses, and sedges.
Bacterial leaf streak (BLS), also known as black chaff, is a common bacterial disease of wheat. The disease is caused by the bacterial species Xanthomonas translucens pv. undulosa. The pathogen is found globally, but is a primary problem in the US in the lower mid-south and can reduce yields by up to 40 percent.[6] BLS is primarily seed-borne and survives in and on the seed, but may also survive in crop residue in the soil in the off-season. During the growing season, the bacteria may transfer from plant to plant by contact, but it is primarily spread by rain, wind and insect contact. The bacteria thrives in moist environments, and produces a cream to yellow bacterial ooze, which, when dry, appears light colored and scale-like, resulting in a streak on the leaves. The invasion of the head of wheat causes bands of necrotic tissue on the awns, which is called Black Chaff.[14] The disease is not easily managed, as there are no pesticides on the market for treatment of the infection. There are some resistant cultivars available, but no seed treatment exists. Some integrated pest management (IPM) techniques may be used to assist with preventing infection although, none will completely prevent the disease.[2]

Bacterial blight of soybean is a widespread disease of soybeans caused by Pseudomonas syringaepv. glycinea.
Silvia Restrepo Restrepo is a Colombian biologist, molecular biologist, and phytopathologist recognised for her investigations into different plant diseases, particularly in the Solanaceae Family. She also leads research about food safety and sustainable agriculture. In 2023, she was appointed as the first woman president of the Boyce Thompson Institute associated with Cornell University in Ithaca, New York. She is the former vice president of research and creation at Universidad de Los Andes.
References
- 1 2 3 4 5 6 7 8 Lozano, J. Carlos (September 1986). "Cassava bacterial blight: a manageable disease" (PDF). Plant Disease. 70 (12): 1089–1093.
- ↑ "Manihot GRIN-Global". npgsweb.ars-grin.gov. Retrieved 2023-01-14.
- 1 2 3 "Cassava bacterial blight (Xanthomonas axonopodis pv. Manihotis)".
- 1 2 Wall, George C. (August 2000). "Bacterial Blight of Mendioka (Cassava) (Xanthomonas campestris pv. manihotis)" (PDF). Agricultural Pests of the Pacific. ADAP 2000-1. ISBN 1-931435-04-9.
- ↑ "Strategic environmental assessment". www.fao.org. Retrieved 2018-12-07.
- ↑ Nukenine, E. N.; Ngeve, J. M.; Dixon, A. G. O. (2002-10-01). "Genotype × environment Effects on Severity of Cassava Bacterial Blight Disease caused by Xanthomonas axonopodis pv. manihotis" (PDF). European Journal of Plant Pathology. 108 (8): 763–770. doi:10.1023/A:1020876019227. ISSN 1573-8469. S2CID 5956093.
- ↑ Lopez, Camilo; Jorge, Véronique; Mosquera, Gloria; Restrepo, Silvia; Verdier, Valérie (2004-11-01). "Recent progress in the characterization of molecular determinants in the Xanthomonas axonopodis pv. manihotis–cassava interaction" (PDF). Plant Molecular Biology. 56 (4): 573–584. doi:10.1007/s11103-004-5044-8. ISSN 1573-5028. PMID 15630621. S2CID 7507168.
- ↑ Martin, Gregory B.; Abramovitch, Robert B. (2005-04-01). "AvrPtoB: A bacterial type III effector that both elicits and suppresses programmed cell death associated with plant immunity". FEMS Microbiology Letters. 245 (1): 1–8. doi: 10.1016/j.femsle.2005.02.025 . ISSN 0378-1097. PMID 15796972.
- ↑ Mansfield, John; Genin, Stephane; Magori, Shimpei; Citovsky, Vitaly; Sriariyanum, Malinee; Ronald, Pamela; Dow, Max; Verdier, Valérie; Beer, Steven V. (2012-08-01). "Top 10 plant pathogenic bacteria in molecular plant pathology". Molecular Plant Pathology. 13 (6): 614–629. doi:10.1111/j.1364-3703.2012.00804.x. ISSN 1364-3703. PMC 6638704 . PMID 22672649.
- 1 2 Castiblanco, Luisa F.; Gil, Juliana; Rojas, Alejandro; Osorio, Daniela; Gutiérrez, Sonia; Muñoz‐Bodnar, Alejandra; Perez‐Quintero, Alvaro L.; Koebnik, Ralf; Szurek, Boris (2013-01-01). "TALE1 from Xanthomonas axonopodis pv. manihotis acts as a transcriptional activator in plant cells and is important for pathogenicity in cassava plants". Molecular Plant Pathology. 14 (1): 84–95. doi:10.1111/j.1364-3703.2012.00830.x. ISSN 1364-3703. PMC 6638846 . PMID 22947214.
- 1 2 Cohn, Megan; Bart, Rebecca S.; Shybut, Mikel; Dahlbeck, Douglas; Gomez, Michael; Morbitzer, Robert; Hou, Bi-Huei; Frommer, Wolf B.; Lahaye, Thomas; Staskawicz, Brian J. (2014). "APS Journals". Molecular Plant-Microbe Interactions. 27 (11): 1186–1198. doi: 10.1094/mpmi-06-14-0161-r . PMID 25083909.
- 1 2 Bernal, Adriana J.; López, Camilo E. (2012-03-01). "Cassava Bacterial Blight: Using Genomics for the Elucidation and Management of an Old Problem". Tropical Plant Biology. 5 (1): 117–126. doi:10.1007/s12042-011-9092-3. ISSN 1935-9764. S2CID 15415306.
- 1 2 Banito, Agnassim (2003). Integrated control of cassava bacterial blight in West Africa in relation to ecozones, host plant resistance and cultural practices. PhD dissertation, University of Hannover, Germany.
- ↑ Okese, K. Afrane (2016-10-20). "Cassava Bacterial Blight Disease: Prevention and control". Agrihome. Retrieved 2018-12-07.
- ↑ Jorge, V.; Fregene, M. A.; Duque, M. C.; Bonierbale, M. W.; Tohme, J.; Verdier, V. (2000). "Genetic mapping of resistance to bacterial blight disease in cassava ( Manihot esculenta Crantz)" (PDF). Theoretical and Applied Genetics. 101 (5–6): 865–872. doi:10.1007/s001220051554. hdl: 10568/42899 . S2CID 12043910.
- ↑ "Cassava". International Institute of Tropical Agriculture.