Related Research Articles

Amyloid-beta precursor protein (APP) is an integral membrane protein expressed in many tissues and concentrated in the synapses of neurons. It functions as a cell surface receptor and has been implicated as a regulator of synapse formation, neural plasticity, antimicrobial activity, and iron export. It is coded for by the gene APP and regulated by substrate presentation. APP is best known as the precursor molecule whose proteolysis generates amyloid beta (Aβ), a polypeptide containing 37 to 49 amino acid residues, whose amyloid fibrillar form is the primary component of amyloid plaques found in the brains of Alzheimer's disease patients.

Amyloid plaques are extracellular deposits of the amyloid beta (Aβ) protein mainly in the grey matter of the brain. Degenerative neuronal elements and an abundance of microglia and astrocytes can be associated with amyloid plaques. Some plaques occur in the brain as a result of aging, but large numbers of plaques and neurofibrillary tangles are characteristic features of Alzheimer's disease. The plaques are highly variable in shape and size; in tissue sections immunostained for Aβ, they comprise a log-normal size distribution curve, with an average plaque area of 400-450 square micrometers (µm²). The smallest plaques, which often consist of diffuse deposits of Aβ, are particularly numerous. Plaques form when Aβ misfolds and aggregates into oligomers and longer polymers, the latter of which are characteristic of amyloid.

A neurodegenerative disease is caused by the progressive loss of structure or function of neurons, in the process known as neurodegeneration. Such neuronal damage may ultimately involve cell death. Neurodegenerative diseases include amyotrophic lateral sclerosis, multiple sclerosis, Parkinson's disease, Alzheimer's disease, Huntington's disease, multiple system atrophy, tauopathies, and prion diseases. Neurodegeneration can be found in the brain at many different levels of neuronal circuitry, ranging from molecular to systemic. Because there is no known way to reverse the progressive degeneration of neurons, these diseases are considered to be incurable; however research has shown that the two major contributing factors to neurodegeneration are oxidative stress and inflammation. Biomedical research has revealed many similarities between these diseases at the subcellular level, including atypical protein assemblies and induced cell death. These similarities suggest that therapeutic advances against one neurodegenerative disease might ameliorate other diseases as well.

Beta-secretase 1, also known as beta-site amyloid precursor protein cleaving enzyme 1, beta-site APP cleaving enzyme 1 (BACE1), membrane-associated aspartic protease 2, memapsin-2, aspartyl protease 2, and ASP2, is an enzyme that in humans is encoded by the BACE1 gene. Expression of BACE1 is observed mainly in neurons.
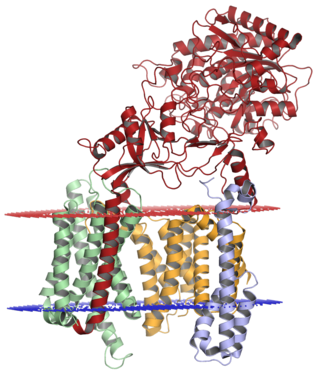
Gamma secretase is a multi-subunit protease complex, itself an integral membrane protein, that cleaves single-pass transmembrane proteins at residues within the transmembrane domain. Proteases of this type are known as intramembrane proteases. The most well-known substrate of gamma secretase is amyloid precursor protein, a large integral membrane protein that, when cleaved by both gamma and beta secretase, produces a short 37-43 amino acid peptide called amyloid beta whose abnormally folded fibrillar form is the primary component of amyloid plaques found in the brains of Alzheimer's disease patients. Gamma secretase is also critical in the related processing of several other type I integral membrane proteins, such as Notch, ErbB4, E-cadherin, N-cadherin, ephrin-B2, or CD44.

Presenilins are a family of related multi-pass transmembrane proteins which constitute the catalytic subunits of the gamma-secretase intramembrane protease protein complex. They were first identified in screens for mutations causing early onset forms of familial Alzheimer's disease by Peter St George-Hyslop. Vertebrates have two presenilin genes, called PSEN1 that codes for presenilin 1 (PS-1) and PSEN2 that codes for presenilin 2 (PS-2). Both genes show conservation between species, with little difference between rat and human presenilins. The nematode worm C. elegans has two genes that resemble the presenilins and appear to be functionally similar, sel-12 and hop-1.
Christine Van Broeckhoven is a Belgian molecular biologist and professor in Molecular genetics at the University of Antwerp. She is also leading the VIB Department of Molecular Genetics, University of Antwerp of the Flanders Institute for Biotechnology (VIB). Christine Van Broeckhoven does research on Alzheimer dementia, bipolar mental disorders and other neurological diseases. Since 1983 she has had her own laboratory for molecular genetics at the University of Antwerp, and since 2005 is focussing her research on neurodegenerative brain diseases. She is an associate editor of the scientific journal Genes, Brain and Behavior.
The Potamkin Prize for Research in Pick's, Alzheimer's, and Related Diseases was established in 1988 and is sponsored by the American Academy of Neurology. The prize is funded through the philanthropy of the Potamkin Foundation. The prize is awarded for achievements on emerging areas of research in Pick's disease, Alzheimer's disease and other dementias.
Sir John Anthony Hardy is a human geneticist and molecular biologist at the Reta Lila Weston Institute of Neurological Studies at University College London with research interests in neurological diseases.
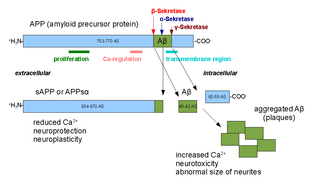
Alpha secretases are a family of proteolytic enzymes that cleave amyloid precursor protein (APP) in its transmembrane region. Specifically, alpha secretases cleave within the fragment that gives rise to the Alzheimer's disease-associated peptide amyloid beta when APP is instead processed by beta secretase and gamma secretase. The alpha-secretase pathway is the predominant APP processing pathway. Thus, alpha-secretase cleavage precludes amyloid beta formation and is considered to be part of the non-amyloidogenic pathway in APP processing. Alpha secretases are members of the ADAM family, which are expressed on the surfaces of cells and anchored in the cell membrane. Several such proteins, notably ADAM10, have been identified as possessing alpha-secretase activity. Upon cleavage by alpha secretases, APP releases its extracellular domain - a fragment known as APPsα - into the extracellular environment in a process known as ectodomain shedding.

Nicastrin, also known as NCSTN, is a protein that in humans is encoded by the NCSTN gene.
APH-1 is a protein gene product originally identified in the Notch signaling pathway in Caenorhabditis elegans as a regulator of the cell-surface localization of nicastrin. APH-1 homologs in other organisms, including humans, have since been identified as components of the gamma secretase complex along with the catalytic subunit presenilin and the regulatory subunits nicastrin and PEN-2. The gamma-secretase complex is a multimeric protease responsible for the intramembrane proteolysis of transmembrane proteins such as the Notch protein and amyloid precursor protein (APP). Gamma-secretase cleavage of APP is one of two proteolytic steps required to generate the peptide known as amyloid beta, whose misfolded form is implicated in the causation of Alzheimer's disease. All of the components of the gamma-secretase complex undergo extensive post-translational modification, especially proteolytic activation; APH-1 and PEN-2 are regarded as regulators of the maturation process of the catalytic component presenilin. APH-1 contains a conserved alpha helix interaction motif glycine-X-X-X-glycine (GXXXG) that is essential to both assembly of the gamma secretase complex and to the maturation of the components.

Presenilin-1(PS-1) is a presenilin protein that in humans is encoded by the PSEN1 gene. Presenilin-1 is one of the four core proteins in the gamma secretase complex, which is considered to play an important role in generation of amyloid beta (Aβ) from amyloid-beta precursor protein (APP). Accumulation of amyloid beta is associated with the onset of Alzheimer's disease.

Presenilin-2 is a protein that is encoded by the PSEN2 gene.
Early-onset Alzheimer's disease (EOAD), also called younger-onset Alzheimer's disease (YOAD), is Alzheimer's disease diagnosed before the age of 65. It is an uncommon form of Alzheimer's, accounting for only 5–10% of all Alzheimer's cases. About 60% have a positive family history of Alzheimer's and 13% of them are inherited in an autosomal dominant manner. Most cases of early-onset Alzheimer's share the same traits as the "late-onset" form and are not caused by known genetic mutations. Little is understood about how it starts.
Intramembrane proteases (IMPs), also known as intramembrane-cleaving proteases (I-CLiPs), are enzymes that have the property of cleaving transmembrane domains of integral membrane proteins. All known intramembrane proteases are themselves integral membrane proteins with multiple transmembrane domains, and they have their active sites buried within the lipid bilayer of cellular membranes. Intramembrane proteases are responsible for proteolytic cleavage in the cell signaling process known as regulated intramembrane proteolysis (RIP).

Karen Elizabeth Keitley Duff is a British scientist known for her work on Alzheimer's disease. Her most notable work focused on the development and characterization of mouse models of Alzheimer's disease amyloid deposition. She became Centre Director of the UK Dementia Research Institute's hub at University College London in spring 2020.
Dennis J. Selkoe is an American physician (neurologist) known for his research into the molecular basis of Alzheimer's disease. In 1985 he became Co-Director of the Center for Neurological Diseases and from 1990, Vincent and Stella Coates Professor of Neurological Diseases at Harvard Medical School. He is also a Fellow of the AAAS and a member of the National Academy of Medicine.
Alzheimer's disease (AD) in the Hispanic/Latino population is becoming a topic of interest in AD research as Hispanics and Latinos are disproportionately affected by Alzheimer's Disease and underrepresented in clinical research. AD is a neurodegenerative disease, characterized by the presence of amyloid-beta plaques and neurofibrillary tangles, that causes memory loss and cognitive decline in its patients. However, pathology and symptoms have been shown to manifest differently in Hispanic/Latinos, as different neuroinflammatory markers are expressed and cognitive decline is more pronounced. Additionally, there is a large genetic component of AD, with mutations in the amyloid precursor protein (APP), Apolipoprotein E APOE), presenilin 1 (PSEN1), bridging Integrator 1 (BIN1), SORL1, and Clusterin (CLU) genes increasing one's risk to develop the condition. However, research has shown these high-risk genes have a different effect on Hispanics and Latinos then they do in other racial and ethnic groups. Additionally, this population experiences higher rates of comorbidities, that increase their risk of developing AD. Hispanics and Latinos also face socioeconomic and cultural factors, such as low income and a language barrier, that affect their ability to engage in clinical trials and receive proper care.

Carol Joyce Jennings is a British campaigner and advocate for research into Alzheimer's Disease. As of 2023 she serves as an honorary Vice-President of the Alzheimer's Society. Through her activism in the 1980s, Jennings brought her family to the attention of researchers studying the disease, which subsequently led to the discovery of the London Mutation. This mutation, found on the Amyloid Precursor Protein (APP) gene located on chromosome 21, marked a significant breakthrough in understanding the genetic basis of Alzheimer's Disease and provided evidence for the development of the 'amyloid hypothesis', which attempts to explain the underlying causes of Alzheimer's Disease.
References
- ↑ "Bart De Strooper". Cure Alzheimer's Fund. Retrieved 7 November 2023.
- ↑ "Bart De Strooper". Crick. 15 September 2023. Retrieved 7 November 2023.
- ↑ "Director announced to lead landmark UK Dementia Research Institute". Alzheimer's society. 14 December 2016. Retrieved 14 December 2016.
- ↑ "Comment: Professor Bart De Strooper steps down as Director of the UK Dementia Research Institute". www.dementiasplatform.uk. Retrieved 7 November 2023.
- ↑ American Academy of Neurology. "2002 Potamkin prize recognizes the ground-breaking work of Alzheimer's disease researchers in Germany, Belgium".
- ↑ "MetLife Foundation Awards for Medical Research in Alzheimer's Disease" (PDF). Archived from the original (PDF) on 13 October 2018.
- ↑ "Home - Lundbeckfonden - The Brain Prize". www.thebrainprize.org. Retrieved 29 July 2018.
- ↑ "Expertscape: Alzheimer Disease, November 2018". expertscape.com. November 2018. Retrieved 15 November 2018.
- De Strooper, B.; Saftig, P.; Craessaerts, K.; Vanderstichele, H.; Guhde, G.; Annaert, W.; von Figura, K.; Van Leuven, F. (1998). "Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein". Nature . 391 (6665): 387–90. doi:10.1038/34910. PMID 9450754. S2CID 4397720.
- De Strooper, B.; Kopan, R.; Annaert, W.; Cupers, P.; Saftig, P.; Craessaerts, K.; Mumm, J. S.; Schroeter, E. H.; Schrijvers, V.; Wolfe, M. S.; Ray, W. J.; Goate, A. (1999). "A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain". Nature . 398 (6727): 518–22. doi:10.1038/19083. PMID 10206645. S2CID 4346474.