Related Research Articles

Raman spectroscopy is a spectroscopic technique typically used to determine vibrational modes of molecules, although rotational and other low-frequency modes of systems may also be observed. Raman spectroscopy is commonly used in chemistry to provide a structural fingerprint by which molecules can be identified.

Rotational spectroscopy is concerned with the measurement of the energies of transitions between quantized rotational states of molecules in the gas phase. The spectra of polar molecules can be measured in absorption or emission by microwave spectroscopy or by far infrared spectroscopy. The rotational spectra of non-polar molecules cannot be observed by those methods, but can be observed and measured by Raman spectroscopy. Rotational spectroscopy is sometimes referred to as pure rotational spectroscopy to distinguish it from rotational-vibrational spectroscopy where changes in rotational energy occur together with changes in vibrational energy, and also from ro-vibronic spectroscopy where rotational, vibrational and electronic energy changes occur simultaneously.
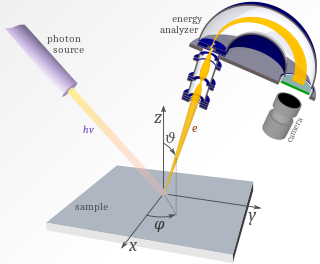
Photoemission spectroscopy (PES), also known as photoelectron spectroscopy, refers to energy measurement of electrons emitted from solids, gases or liquids by the photoelectric effect, in order to determine the binding energies of electrons in the substance. The term refers to various techniques, depending on whether the ionization energy is provided by X-ray, XUV or UV photons. Regardless of the incident photon beam, however, all photoelectron spectroscopy revolves around the general theme of surface analysis by measuring the ejected electrons.

Molecular physics is the study of the physical properties of molecules and molecular dynamics. The field overlaps significantly with physical chemistry, chemical physics, and quantum chemistry. It is often considered as a sub-field of atomic, molecular, and optical physics. Research groups studying molecular physics are typically designated as one of these other fields. Molecular physics addresses phenomena due to both molecular structure and individual atomic processes within molecules. Like atomic physics, it relies on a combination of classical and quantum mechanics to describe interactions between electromagnetic radiation and matter. Experiments in the field often rely heavily on techniques borrowed from atomic physics, such as spectroscopy and scattering.
Microwave spectroscopy is the spectroscopy method that employs microwaves, i.e. electromagnetic radiation at GHz frequencies, for the study of matter.
Fluorescence correlation spectroscopy (FCS) is a statistical analysis, via time correlation, of stationary fluctuations of the fluorescence intensity. Its theoretical underpinning originated from L. Onsager's regression hypothesis. The analysis provides kinetic parameters of the physical processes underlying the fluctuations. One of the interesting applications of this is an analysis of the concentration fluctuations of fluorescent particles (molecules) in solution. In this application, the fluorescence emitted from a very tiny space in solution containing a small number of fluorescent particles (molecules) is observed. The fluorescence intensity is fluctuating due to Brownian motion of the particles. In other words, the number of the particles in the sub-space defined by the optical system is randomly changing around the average number. The analysis gives the average number of fluorescent particles and average diffusion time, when the particle is passing through the space. Eventually, both the concentration and size of the particle (molecule) are determined. Both parameters are important in biochemical research, biophysics, and chemistry.
Ultrafast laser spectroscopy is a spectroscopic technique that uses ultrashort pulse lasers for the study of dynamics on extremely short time scales. Different methods are used to examine the dynamics of charge carriers, atoms, and molecules. Many different procedures have been developed spanning different time scales and photon energy ranges; some common methods are listed below.
This page deals with the electron affinity as a property of isolated atoms or molecules. Solid state electron affinities are not listed here.
Giacinto Scoles is a European and North American chemist and physicist who is best known for his pioneering development of molecular beam methods for the study of weak van der Waals forces between atoms, molecules, and surfaces. He developed the cryogenic bolometer as a universal detector of atomic and molecule beams that not only can detect a small flux of molecules, but also responds to the internal energy of the molecules. This is the basis for the optothermal spectroscopy technique which Scoles and others have used to obtain very high signal-to noise and high resolution ro-vibrational spectra.
Photofragment ion imaging or, more generally, Product Imaging is an experimental technique for making measurements of the velocity of product molecules or particles following a chemical reaction or the photodissociation of a parent molecule. The method uses a two-dimensional detector, usually a microchannel plate, to record the arrival positions of state-selected ions created by resonantly enhanced multi-photon ionization (REMPI). The first experiment using photofragment ion imaging was performed by David W Chandler and Paul L Houston in 1987 on the phototodissociation dynamics of methyl iodide (iodomethane, CH3I).
Xenon monochloride (XeCl) is an exciplex which is used in excimer lasers and excimer lamps emitting near ultraviolet light at 308 nm. It is most commonly used in medicine. Xenon monochloride was first synthesized in the 1960s. Its kinetic scheme is very complex and its state changes occur on a nanosecond timescale. In the gaseous state, at least two kinds of xenon monochloride are known: XeCl and Xe
2Cl, whereas complex aggregates form in the solid state in noble gas matrices. The excited state of xenon resembles halogens and it reacts with them to form excited molecular compounds.
Robert Tycko is an American biophysicist whose research primarily involves solid state NMR, including the development of new methods and applications to various areas of physics, chemistry, and biology. He is a member of the Laboratory of Chemical Physics in the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health in Bethesda, Maryland, USA. He was formerly a member of the Physical Chemistry Research and Materials Chemistry Research departments of AT&T Bell Labs in Murray Hill, New Jersey. His work has contributed to our understanding of geometric phases in spectroscopy, physical properties of fullerenes, skyrmions in 2D electron systems, protein folding, and amyloid fibrils associated with Alzheimer’s disease and prions.

Calcium monohydride is a molecule composed of calcium and hydrogen with formula CaH. It can be found in stars as a gas formed when calcium atoms are present with hydrogen atoms.

Martin Quack is a German physical chemist and spectroscopist; he is a professor at ETH Zürich.

Bidyendu Mohan Deb is an Indian theoretical chemist, chemical physicist and a professor at the Indian Institute of Science Education and Research, Kolkata (IISER). he is known for his studies in theoretical chemistry and chemical physics. He is an elected fellow of the International Union of Pure and Applied Chemistry, The World Academy of Sciences, Indian National Science Academy and the Indian Academy of Sciences. The Council of Scientific and Industrial Research, the apex agency of the Government of India for scientific research, awarded him the Shanti Swarup Bhatnagar Prize for Science and Technology, one of the highest Indian science awards, in 1981, for his contributions to chemical sciences.

R. J. Dwayne Miller is a Canadian chemist and a professor at the University of Toronto. His focus is in physical chemistry and biophysics. He is most widely known for his work in ultrafast laser science, time-resolved spectroscopy, and the development of new femtosecond electron sources. His research has enabled real-time observation of atomic motions in materials during chemical processes and has shed light on the structure-function correlation that underlies biology.

Diargon or the argon dimer is a molecule containing two argon atoms. Normally, this is only very weakly bound together by van der Waals forces. However, in an excited state, or ionised state, the two atoms can be more tightly bound together, with significant spectral features. At cryogenic temperatures, argon gas can have a few percent of diargon molecules.
Hrvoje Petek is a Croatian-born American physicist and the Richard King Mellon Professor of Physics and Astronomy, at the University of Pittsburgh, where he is also a professor of chemistry.
Tamar Seideman is the Dow Chemical Company Professor of Chemistry and Professor of Physics at Northwestern University. She specialises in coherence spectroscopies and coherent control in isolated molecules and dissipative media as well as in ultrafast nanoplasmonics, current-driven phenomena in nanoelectronics and mathematical models.
David Leslie Andrews,, is a British scientist appointed as Professor of Chemical Physics at the University of East Anglia, where he was the Head of Chemical Sciences and Physics, from 1996 to 1999.