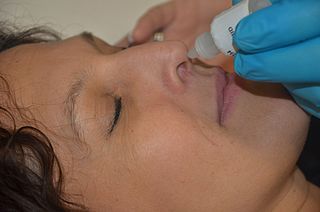
A topical medication is a medication that is applied to a particular place on or in the body. Most often topical medication means application to body surfaces such as the skin or mucous membranes to treat ailments via a large range of classes including creams, foams, gels, lotions, and ointments. Many topical medications are epicutaneous, meaning that they are applied directly to the skin. Topical medications may also be inhalational, such as asthma medications, or applied to the surface of tissues other than the skin, such as eye drops applied to the conjunctiva, or ear drops placed in the ear, or medications applied to the surface of a tooth. The word topical derives from Greek τοπικόςtopikos, "of a place".
Shire plc was a UK-founded Jersey-registered specialty biopharmaceutical company. Originating in the United Kingdom with an operational base in the United States, its brands and products included Vyvanse, Lialda, and Adderall XR. Shire was acquired by Takeda Pharmaceutical Company on 8 January 2019.

Gilead Sciences, Inc. is an American biopharmaceutical company headquartered in Foster City, California that focuses on researching and developing antiviral drugs used in the treatment of HIV/AIDS, hepatitis B, hepatitis C, influenza, and COVID-19, including ledipasvir/sofosbuvir and sofosbuvir. Gilead is a member of the NASDAQ Biotechnology Index and the S&P 500.
Adalimumab, sold under the brand name Humira and others, is a disease-modifying antirheumatic drug and monoclonal antibody used to treat rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn's disease, ulcerative colitis, plaque psoriasis, hidradenitis suppurativa, and uveitis. It is administered by subcutaneous injection. It works by inactivating tumor necrosis factor-alpha (TNFα).
C.H. Boehringer Sohn AG & Co. KG is the parent company of the Boehringer Ingelheim group, which was founded in 1885 by Albert Boehringer (1861–1939) in Ingelheim am Rhein, Germany. As of 2018, Boehringer Ingelheim is one of the world's largest pharmaceutical companies, and the largest private one. Headquartered in Ingelheim, it operates globally with 146 affiliates and more than 47,700 employees. Unlike most large pharmaceutical companies which are listed, the company is private and fully owned by the Boehringer, Liebrecht and von Baumbach families. The company's key areas of interest are: respiratory diseases, metabolism, immunology, oncology and diseases of the central nervous system. Boehringer Ingelheim is a full member of the European Federation of Pharmaceutical Industries and Associations (EFPIA). The corporate logo of Boehringer Ingelheim depicts a stylized rendition of the central section of the imperial palace of Charlemagne.

Clindamycin/benzoyl peroxide is a topical gel used for the treatment of acne. It is a fixed-dose combination of clindamycin, as the phosphate, an antibiotic, and benzoyl peroxide, an antiseptic.
Endo International plc is an American Irish-domiciled generics and specialty branded pharmaceutical company that generated over 93% of its 2017 sales from the U.S. healthcare system. While Endo's management, operations, and customers are almost exclusively U.S.–based, in 2013 Endo executed a corporate tax inversion to Ireland to avoid U.S. corporate taxes on their U.S. drug sales, and to avail of Ireland's corporate tax system.

Amcinonide is a topical glucocorticoid used to treat itching, redness and swelling associated with several dermatologic conditions such as atopic dermatitis and allergic contact dermatitis. Amcinonide can also be classified as a multi-functional small molecule corticosteroid, which has been approved by the FDA and is currently marketed as an ointment, lotion, or cream. It acts as both a transcription factor for responses to glucocorticoids and modulator for other transcription factors while also regulating phospholipase A2 activity.

Mallinckrodt Pharmaceuticals is an American-Irish domiciled manufacturer of specialty pharmaceuticals, generic drugs and imaging agents. In 2017, it generated 90% of its sales from the U.S. healthcare system. While Mallinckrodt is headquartered in Ireland for tax purposes, its operational headquarters are in the U.S. Mallinckrodt's 2013 tax inversion to Ireland drew controversy when it was shown Acthar was Medicaid's most expensive drug.

Phonophoresis, also known as sonophoresis, is the method of using ultrasound waves to increase skin permeability in order to improve the effectiveness of transdermal drug delivery. This method intersects drug delivery and ultrasound therapy. By assisting transdermal drug delivery, phonophoresis can be a painless treatment and an alternative to a needle.
A patent thicket is "an overlapping set of patent rights" which requires innovators to reach licensing deals for multiple patents. This concept has negative connotations and has been described as "a dense web of overlapping intellectual property rights that a company must hack its way through in order to actually commercialize new technology".

The National Organization for Rare Disorders (NORD) is an American non-profit organization aiming to provide support for individuals with rare diseases by advocating and funding research, education, and networking among service providers. It was founded in 1983 by Abbey Meyers, along with individuals and rare diseases leaders of rare disease support groups, and it is a 501(c)(3) tax exempt organization.
Repros Therapeutics Inc. (Nasdaq: RPRX), was a US-based development stage biopharmaceutical company headquartered in The Woodlands, Texas. Founded in 1987 as Zonagen, it focused on the development of oral small molecule drugs to address major unmet medical needs in male and female health. Joseph S. Podolski was the CEO of this company.

A transdermal analgesic or pain relief patch is a medicated adhesive patch used to relieve minor to severe pain. There are many types of analgesic patches based on the main ingredients in the patches. These include patches containing counterirritants, which are used to treat mild to moderate pain, and patches containing opioids such as buprenorphine and fentanyl, used to relieve moderate to severe pain. Fentanyl is often used for opioid-tolerant patients. Nitroglycerin, also known as glyceryl trinitrate (GTN), a medication used for heart failure, high blood pressure, anal fissures, painful periods, and to treat and prevent chest pain, can also be found in patches. Beyond these are patches that contain drugs such as diclofenac and lidocaine and various other drugs. The main purpose of transdermal analgesic patches are to administer drugs in a more viable way to patients, as opposed to oral consumption or intravenous administration such as an injection.
AbbVie Inc. is an American pharmaceutical company headquartered in North Chicago, Illinois. It is ranked sixth on the list of largest biomedical companies by revenue. In 2023, the company's seat in Forbes Global 2000 was 74. The company's primary product is Humira (adalimumab), administered via injection. It is approved to treat autoimmune diseases including rheumatoid arthritis, Crohn's disease, plaque psoriasis, and ulcerative colitis.
Assertio Therapeutics, Inc. is an American specialty pharmaceutical company. It mainly markets products for treatment in neurology, pain and diseases of the central nervous system. Depomed was founded in 1995 and is headquartered in Newark, California. It is a publicly traded company on NASDAQ, with several products approved by the United States Food and Drug Administration (FDA). On August 15, 2018, the company announced its name change from Depomed, Inc., to Assertio Therapeutics, Inc. As of 2019, Assertio markets three products approved by the FDA: Gralise, Cambia, and Zipsor.
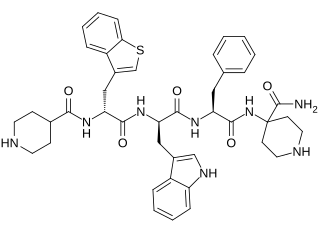
Relamorelin is a synthetic peptide, centrally penetrant, selective agonist of the ghrelin/growth hormone secretagogue receptor (GHSR) which is under development by Allergan pharmaceuticals for the treatment of diabetic gastroparesis, chronic idiopathic constipation, and anorexia nervosa. It is a pentapeptide and an analogue of ghrelin with improved potency and pharmacokinetics. In humans, relamorelin produces increases in plasma growth hormone, prolactin, and cortisol levels, and, like other GHSR agonists, increases appetite. As of June 2015, relamorelin is in phase II clinical trials for diabetic gastroparesis and constipation. The United States Food and Drug Administration (FDA) has granted Fast Track designation to relamorelin for diabetic gastroparesis. The development of the drug is uncertain as the most recent mention of it was in a 2019 SEC filing from the drug manufacturer lists the drug's expected launch year as 2024, but not in subsequent filings or press releases.
Allergan plc is an American, Irish-domiciled pharmaceutical company that acquires, develops, manufactures and markets brand name drugs and medical devices in the areas of medical aesthetics, eye care, central nervous system, and gastroenterology. The company is the maker of Botox.

Ethosomes are phospholipid nanovesicles used for dermal and transdermal delivery of molecules. Ethosomes were developed by Touitou et al.,1997, as additional novel lipid carriers composed of ethanol, phospholipids, and water. They are reported to improve the skin delivery of various drugs. Ethanol is an efficient permeation enhancer that is believed to act by affecting the intercellular region of the stratum corneum. Ethosomes are soft malleable vesicles composed mainly of phospholipids, ethanol, and water. These soft vesicles represent novel vesicles carriers for enhanced delivery through the skin. The size of the ethosomes vesicles can be modulated from tens of nanometers to microns.
Cerevel Therapeutics Holdings, Inc. is a biotechnology and pharmaceuticals company focused on the development of novel therapies for mental and neurological illnesses. Cerevel was established in October 2018, and is headquartered in Cambridge, Massachusetts.










