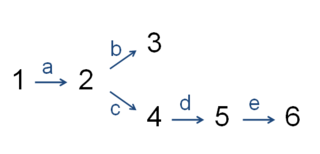In biochemistry, a metabolic pathway is a linked series of chemical reactions occurring within a cell. The reactants, products, and intermediates of an enzymatic reaction are known as metabolites, which are modified by a sequence of chemical reactions catalyzed by enzymes. In most cases of a metabolic pathway, the product of one enzyme acts as the substrate for the next. However, side products are considered waste and removed from the cell.
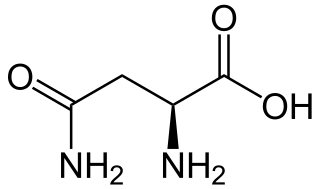
Asparagine is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group, an α-carboxylic acid group, and a side chain carboxamide, classifying it as a polar, aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it. It is encoded by the codons AAU and AAC.
Lipoamide is a trivial name for 6,8-dithiooctanoic amide. It is the functional form of lipoic acid, i.e the carboxyl group is attached to protein via an amine with an amide linkage. Illustrative of the biochemical role of lipoamide is in the conversion of pyruvate to acetyl lipoamide.

A macromolecule is a very large molecule important to biological processes, such as a protein or nucleic acid. It is composed of thousands of covalently bonded atoms. Many macromolecules are polymers of smaller molecules called monomers. The most common macromolecules in biochemistry are biopolymers and large non-polymeric molecules such as lipids, nanogels and macrocycles. Synthetic fibers and experimental materials such as carbon nanotubes are also examples of macromolecules.

Acetyl-CoA is a molecule that participates in many biochemical reactions in protein, carbohydrate and lipid metabolism. Its main function is to deliver the acetyl group to the citric acid cycle to be oxidized for energy production.

Anabolism is the set of metabolic pathways that construct macromolecules like DNA or RNA from smaller units. These reactions require energy, known also as an endergonic process. Anabolism is the building-up aspect of metabolism, whereas catabolism is the breaking-down aspect. Anabolism is usually synonymous with biosynthesis.

Guanosine-5'-triphosphate (GTP) is a purine nucleoside triphosphate. It is one of the building blocks needed for the synthesis of RNA during the transcription process. Its structure is similar to that of the guanosine nucleoside, the only difference being that nucleotides like GTP have phosphates on their ribose sugar. GTP has the guanine nucleobase attached to the 1' carbon of the ribose and it has the triphosphate moiety attached to ribose's 5' carbon.

Transcriptional modification or co-transcriptional modification is a set of biological processes common to most eukaryotic cells by which an RNA primary transcript is chemically altered following transcription from a gene to produce a mature, functional RNA molecule that can then leave the nucleus and perform any of a variety of different functions in the cell. There are many types of post-transcriptional modifications achieved through a diverse class of molecular mechanisms.
The branched-chain α-ketoacid dehydrogenase complex is a multi-subunit complex of enzymes that is found on the mitochondrial inner membrane. This enzyme complex catalyzes the oxidative decarboxylation of branched, short-chain alpha-ketoacids. BCKDC is a member of the mitochondrial α-ketoacid dehydrogenase complex family, which also includes pyruvate dehydrogenase and alpha-ketoglutarate dehydrogenase, key enzymes that function in the Krebs cycle.
A holoprotein or conjugated protein is an apoprotein combined with its prosthetic group.

An oxyanion hole is a pocket in the active site of an enzyme that stabilizes transition state negative charge on a deprotonated oxygen or alkoxide. The pocket typically consists of backbone amides or positively charged residues. Stabilising the transition state lowers the activation energy necessary for the reaction, and so promotes catalysis. For example, proteases such as chymotrypsin contain an oxyanion hole to stabilise the tetrahedral intermediate anion formed during proteolysis and protects substrate's negatively charged oxygen from water molecules. Additionally, it may allow for insertion or positioning of a substrate, which would suffer from steric hindrance if it could not occupy the hole. Enzymes that catalyse multi-step reactions can have multiple oxyanion holes that stabilise different transition states in the reaction.
Trisaccharides are oligosaccharides composed of three monosaccharides with two glycosidic bonds connecting them. Similar to the disaccharides, each glycosidic bond can be formed between any hydroxyl group on the component monosaccharides. Even if all three component sugars are the same, different bond combinations (regiochemistry) and stereochemistry result in trisaccharides that are diastereoisomers with different chemical and physical properties.
In chemistry, de novo synthesis is the synthesis of complex molecules from simple molecules such as sugars or amino acids, as opposed to recycling after partial degradation. For example, nucleotides are not needed in the diet as they can be constructed from small precursor molecules such as formate and aspartate. Methionine, on the other hand, is needed in the diet because while it can be degraded to and then regenerated from homocysteine, it cannot be synthesized de novo.

Fructose 6-phosphate is a derivative of fructose, which has been phosphorylated at the 6-hydroxy group. It is one of several possible fructosephosphates. The β-D-form of this compound is very common in cells. The great majority of glucose is converted to fructose 6-phosphate upon entering a cell. Fructose is predominantly converted to fructose 1-phosphate by fructokinase following cellular import.

Lubert Stryer was an American academic who was the Emeritus Mrs. George A. Winzer Professor of Cell Biology, at Stanford University School of Medicine. His research over more than four decades had been centered on the interplay of light and life. In 2007 he received the National Medal of Science from President Bush at a ceremony at the White House for elucidating the biochemical basis of signal amplification in vision, pioneering the development of high density microarrays for genetic analysis, and authoring the standard undergraduate biochemistry textbook, Biochemistry. It is now in its tenth edition and also edited by Jeremy Berg, Justin Hines, John L. Tymoczko and Gregory J. Gatto, Jr.

A glucogenic amino acid is an amino acid that can be converted into glucose through gluconeogenesis. This is in contrast to the ketogenic amino acids, which are converted into ketone bodies.

Jeremy Mark Berg was founding director of the University of Pittsburgh's Institute for Personalized Medicine. He holds positions as Associate Senior Vice Chancellor for Science Strategy and Planning and Professor of Computational and Systems Biology at the University of Pittsburgh. From 2016 to 2019, Berg was editor in chief of the Science journals.
Storage proteins serve as biological reserves of metal ions and amino acids, used by organisms. They are found in plant seeds, egg whites, and milk.

Biophysical chemistry is a physical science that uses the concepts of physics and physical chemistry for the study of biological systems. The most common feature of the research in this subject is to seek an explanation of the various phenomena in biological systems in terms of either the molecules that make up the system or the supra-molecular structure of these systems. Apart from the biological applications, recent research showed progress in the medical field as well.
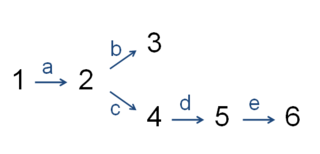
In enzymology, the committed step is an effectively irreversible enzymatic reaction that occurs at a branch point during the biosynthesis of some molecules. As the name implies, after this step, the molecules are "committed" to the pathway and will ultimately end up in the pathway's final product. The first committed step should not be confused with the rate-limiting step, which is the step with the highest flux control coefficient. It is rare that the first committed step is in fact the rate-determining step.