
Biomedical engineering (BME) or medical engineering is the application of engineering principles and design concepts to medicine and biology for healthcare applications. BME is also traditionally logical sciences to advance health care treatment, including diagnosis, monitoring, and therapy. Also included under the scope of a biomedical engineer is the management of current medical equipment in hospitals while adhering to relevant industry standards. This involves procurement, routine testing, preventive maintenance, and making equipment recommendations, a role also known as a Biomedical Equipment Technician (BMET) or as a clinical engineer.

The Takeda Pharmaceutical Company Limited is a Japanese multinational pharmaceutical company. It is the third largest pharmaceutical company in Asia, behind Sinopharm and Shanghai Pharmaceuticals, and one of the top 20 largest pharmaceutical companies in the world by revenue. The company has over 49,578 employees worldwide and achieved US$19.299 billion in revenue during the 2018 fiscal year. The company is focused on oncology, rare diseases, neuroscience, gastroenterology, plasma-derived therapies and vaccines. Its headquarters is located in Chuo-ku, Osaka, and it has an office in Nihonbashi, Chuo, Tokyo. In January 2012, Fortune Magazine ranked the Takeda Oncology Company as one of the 100 best companies to work for in the United States. As of 2015, Christophe Weber was appointed as the CEO and president of Takeda.
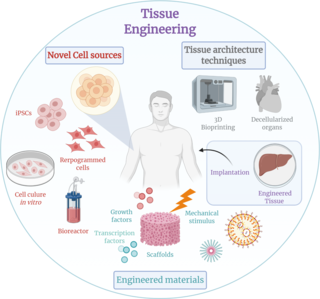
Tissue engineering is a biomedical engineering discipline that uses a combination of cells, engineering, materials methods, and suitable biochemical and physicochemical factors to restore, maintain, improve, or replace different types of biological tissues. Tissue engineering often involves the use of cells placed on tissue scaffolds in the formation of new viable tissue for a medical purpose, but is not limited to applications involving cells and tissue scaffolds. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance, it can is considered as a field of its own.

In medicine, a stent is a tube usually constructed of a metallic alloy or a polymer. It is inserted into the lumen of an anatomic vessel or duct to keep the passageway open. Stenting refers to the placement of a stent. The word "stent" is also used as a verb to describe the placement of such a device, particularly when a disease such as atherosclerosis has pathologically narrowed a structure such as an artery.

Boston Scientific Corporation (BSC), headquartered in Watertown, Massachusetts and incorporated in Delaware, is a biomedical/biotechnology engineering firm and multinational manufacturer of medical devices used in interventional medical specialties, including interventional radiology, interventional cardiology, peripheral interventions, neuromodulation, neurovascular intervention, electrophysiology, cardiac surgery, vascular surgery, endoscopy, oncology, urology and gynecology. Boston Scientific is widely known for the development of the Taxus Stent, a drug-eluting stent which is used to open clogged arteries. With the full acquisition of Cameron Health in June 2012, the company also became notable for offering a minimally invasive implantable cardioverter-defibrillator (ICD) which they call the EMBLEM subcutaneous implantable defibrillator (S-ICD).

Biocon Limited is an Indian biopharmaceutical company based in Bangalore. It was founded by Kiran Mazumdar-Shaw in 1978. The company manufactures generic active pharmaceutical ingredients (APIs) that are sold in approximately 120 countries, including the United States and Europe. It also manufactures novel biologics as well as biosimilar insulins and antibodies, which are sold in India as branded formulations. Biocon's biosimilar products are also sold in both bulk and formulation forms in several emerging markets.

Robert Samuel Langer Jr. FREng is an American biotechnologist, businessman, chemical engineer, chemist, and inventor. He is one of the nine Institute Professors at the Massachusetts Institute of Technology.

Organ printing utilizes techniques similar to conventional 3D printing where a computer model is fed into a printer that lays down successive layers of plastics or wax until a 3D object is produced. In the case of organ printing, the material being used by the printer is a biocompatible plastic. The biocompatible plastic forms a scaffold that acts as the skeleton for the organ that is being printed. As the plastic is being laid down, it is also seeded with human cells from the patient's organ that is being printed for. After printing, the organ is transferred to an incubation chamber to give the cells time to grow. After a sufficient amount of time, the organ is implanted into the patient.

A drug-eluting stent (DES) is a tube made of a mesh-like material used to treat narrowed arteries in medical procedures both mechanically and pharmacologically. DES is inserted into a narrowed artery using a balloon. Once the balloon inside the stent is inflated, the stent expands, pushing against the artery wall, keeping the artery open, thereby improving blood flow. The mesh design allows cells to grow through and around it, securing it in place.
Terumo Corporation was founded in 1921 as Red Line Thermometer Corporation by a group of medical scientists led by Dr. Kitasato Shibasaburō to produce medical thermometers in Japan.

Zotarolimus is an immunosuppressant. It is a semi-synthetic derivative of sirolimus (rapamycin). It was designed for use in stents with phosphorylcholine as a carrier. Zotarolimus, or ABT-578, was originally used on Abbott's coronary stent platforms to reduce early inflammation and restenosis; however, Zotarolimus failed Abbott's primary endpoint to bring their stent/drug delivery system to market. The drug was sold/distributed to Medtronic for use on their stent platforms, which is the same drug they use today. Coronary stents reduce early complications and improve late clinical outcomes in patients needing interventional cardiology. The first human coronary stent implantation was first performed in 1986 by Puel et al. However, there are complications associated with stent use, development of thrombosis which impedes the efficiency of coronary stents, haemorrhagic and restenosis complications are problems associated with stents.
Nitinol biocompatibility is an important factor in biomedical applications. Nitinol (NiTi), which is formed by alloying nickel and titanium, is a shape-memory alloy with superelastic properties more similar to that of bone, when compared to stainless steel, another commonly used biomaterial. Biomedical applications that utilize nitinol include stents, heart valve tools, bone anchors, staples, septal defect devices and implants. It is a commonly used biomaterial especially in the development of stent technology.

ARIAD Pharmaceuticals, Inc. was an American oncology company, now part of Takeda Oncology, which was founded in 1991 by Harvey J. Berger, M.D. and headquartered in Cambridge, Massachusetts. ARIAD engaged in the discovery, development, and commercialization of medicines for cancer patients.

Donald E. Ingber is an American cell biologist and bioengineer. He is the founding director of the Wyss Institute for Biologically Inspired Engineering at Harvard University, the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children's Hospital, and Professor of Bioengineering at the Harvard John A. Paulson School of Engineering and Applied Sciences. He is also a member of the American Institute for Medical and Biological Engineering, the National Academy of Engineering, the National Academy of Medicine, the National Academy of Inventors, and the American Academy of Arts and Sciences.

Cell encapsulation is a possible solution to graft rejection in tissue engineering applications. Cell microencapsulation technology involves immobilization of cells within a polymeric semi-permeable membrane. It permits the bidirectional diffusion of molecules such as the influx of oxygen, nutrients, growth factors etc. essential for cell metabolism and the outward diffusion of waste products and therapeutic proteins. At the same time, the semi-permeable nature of the membrane prevents immune cells and antibodies from destroying the encapsulated cells, regarding them as foreign invaders.
AbbVie Inc. is an American pharmaceutical company headquartered in North Chicago, Illinois. It is ranked sixth on the list of largest biomedical companies by revenue. In 2023, the company's seat in Forbes Global 2000 was 74. The company's primary product is Humira (adalimumab), administered via injection. It is approved to treat autoimmune diseases including rheumatoid arthritis, Crohn's disease, plaque psoriasis, and ulcerative colitis.
BTG Limited is an international specialist healthcare company that is developing and commercialising products targeting critical care, cancer and other disorders. The current name was adopted when the British Technology Group changed its name on 27 May 1998. BTG was a constituent of the FTSE 250 Index until it was acquired by Boston Scientific in August 2019.

Yolonda Lorig Colson is an American thoracic surgeon, working in Boston, who was the 103rd president and first female president of the American Association for Thoracic Surgery (AATS), succeeding Shaf Keshavjee, MD and preceding Lars G. Svensson, MD, PhD. Colson is the Chief of the Division of Thoracic Surgery at Massachusetts General Hospital, Hermes C. Grillo Professor in Thoracic Surgery, and Professor of Surgery at Harvard Medical School. Colson is an Officer and Exam Chair for the American Board of Thoracic Surgery. She is also a collaborator of the Grinstaff Group.
Bioprinting drug delivery is a method of using the three-dimensional printing of biomaterials through an additive manufacturing technique to develop drug delivery vehicles that are biocompatible tissue-specific hydrogels or implantable devices. 3D bioprinting uses printed cells and biological molecules to manufacture tissues, organs, or biological materials in a scaffold-free manner that mimics living human tissue to provide localized and tissue-specific drug delivery, allowing for targeted disease treatments with scalable and complex geometry.

Drug eluting implants encompass a wide range of bioactive implants that can be placed in or near a tissue to provide a controlled, sustained or on demand release of drug while overcoming barriers associated with traditional oral and intravenous drug administration, such as limited bioavailability, metabolism, and toxicity. These implants can be used to treat location-specific and surrounding illness and commonly use 3D printing technologies to achieve individualized implants for patients.















