Related Research Articles
Microfluidics refers to the behaviour, precise control, and manipulation of fluids that are geometrically constrained to a small scale at which capillary penetration governs mass transport. It is a multidisciplinary field that involves engineering, physics, chemistry, biochemistry, nanotechnology, and biotechnology. It has practical applications in the design of systems that process low volumes of fluids to achieve multiplexing, automation, and high-throughput screening. Microfluidics emerged in the beginning of the 1980s and is used in the development of inkjet printheads, DNA chips, lab-on-a-chip technology, micro-propulsion, and micro-thermal technologies.

Tissue engineering is the use of a combination of cells, engineering, and materials methods, and suitable biochemical and physicochemical factors to improve or replace biological tissues. Tissue engineering involves the use of a tissue scaffold for the formation of new viable tissue for a medical purpose. While it was once categorized as a sub-field of biomaterials, having grown in scope and importance it can be considered as a field in its own.

Digital microfluidics (DMF) is another platform for lab-on-a-chip systems that is based upon the manipulation of microdroplets. Droplets are dispensed, moved, stored, mixed, reacted, or analyzed on a platform with a set of insulated electrodes. Digital microfluidics can be used together with analytical analysis procedures such as mass spectrometry, colorimetry, electrochemical, and electrochemiluminescense.

A bioreactor refers to any manufactured device or system that supports a biologically active environment. In one case, a bioreactor is a vessel in which a chemical process is carried out which involves organisms or biochemically active substances derived from such organisms. This process can either be aerobic or anaerobic. These bioreactors are commonly cylindrical, ranging in size from litres to cubic metres, and are often made of stainless steel.
The integrated nanoliter system is a measuring, separating, and mixing device that is able to measure fluids to the nanoliter, mix different fluids for a specific product, and separate a solution into simpler solutions.
A bioprocess is a specific process that uses complete living cells or their components to obtain desired products.
Fed-batch culture is, in the broadest sense, defined as an operational technique in biotechnological processes where one or more nutrients (substrates) are fed (supplied) to the bioreactor during cultivation and in which the product(s) remain in the bioreactor until the end of the run. An alternative description of the method is that of a culture in which "a base medium supports initial cell culture and a feed medium is added to prevent nutrient depletion". It is also a type of semi-batch culture. In some cases, all the nutrients are fed into the bioreactor. The advantage of the fed-batch culture is that one can control concentration of fed-substrate in the culture liquid at arbitrarily desired levels.

The Sartorius group is an international pharmaceutical and laboratory equipment supplier, covering the segments of Bioprocess Solutions and Lab Products & Services.
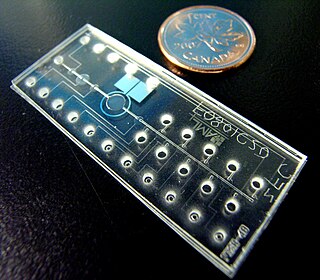
Bio-MEMS is an abbreviation for biomedical microelectromechanical systems. Bio-MEMS have considerable overlap, and is sometimes considered synonymous, with lab-on-a-chip (LOC) and micro total analysis systems (μTAS). Bio-MEMS is typically more focused on mechanical parts and microfabrication technologies made suitable for biological applications. On the other hand, lab-on-a-chip is concerned with miniaturization and integration of laboratory processes and experiments into single chips. In this definition, lab-on-a-chip devices do not strictly have biological applications, although most do or are amenable to be adapted for biological purposes. Similarly, micro total analysis systems may not have biological applications in mind, and are usually dedicated to chemical analysis. A broad definition for bio-MEMS can be used to refer to the science and technology of operating at the microscale for biological and biomedical applications, which may or may not include any electronic or mechanical functions. The interdisciplinary nature of bio-MEMS combines material sciences, clinical sciences, medicine, surgery, electrical engineering, mechanical engineering, optical engineering, chemical engineering, and biomedical engineering. Some of its major applications include genomics, proteomics, molecular diagnostics, point-of-care diagnostics, tissue engineering, single cell analysis and implantable microdevices.
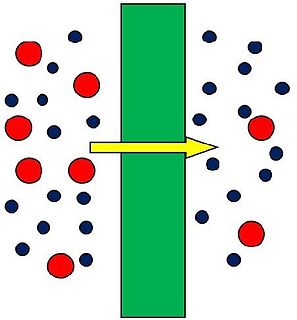
A membrane is a selective barrier; it allows some things to pass through but stops others. Such things may be molecules, ions, or other small particles. Biological membranes include cell membranes ; nuclear membranes, which cover a cell nucleus; and tissue membranes, such as mucosae and serosae. Synthetic membranes are made by humans for use in laboratories and industry.

Nanofountain probe (NFP) is a device for 'drawing' micropatterns of liquid chemicals at extremely small resolution. An NFP contains a cantilevered micro-fluidic device terminated in a nanofountain. The embedded microfluidics facilitates rapid and continuous delivery of molecules from the on-chip reservoirs to the fountain tip. When the tip is brought into contact with the substrate, a liquid meniscus forms, providing a path for molecular transport to the substrate. By controlling the geometry of the meniscus through hold time and deposition speed, various inks and biomolecules could be patterned on a surface, with sub 100 nm resolution.
Microfluidic whole genome haplotyping is a technique for the physical separation of individual chromosomes from a metaphase cell followed by direct resolution of the haplotype for each allele.
A single-use bioreactor or disposable bioreactor is a bioreactor with a disposable bag instead of a culture vessel. Typically, this refers to a bioreactor in which the lining in contact with the cell culture will be plastic, and this lining is encased within a more permanent structure. Commercial single-use bioreactors have been available since the end of the 1990s and are now made by several well-known producers.
An organ-on-a-chip (OOC) is a multi-channel 3-D microfluidic cell culture chip that simulates the activities, mechanics and physiological response of entire organs and organ systems, a type of artificial organ. It constitutes the subject matter of significant biomedical engineering research, more precisely in bio-MEMS. The convergence of labs-on-chips (LOCs) and cell biology has permitted the study of human physiology in an organ-specific context, introducing a novel model of in vitro multicellular human organisms. One day, they will perhaps abolish the need for animals in drug development and toxin testing.
A 3D cell culture is an artificially created environment in which biological cells are permitted to grow or interact with their surroundings in all three dimensions. Unlike 2D environments, a 3D cell culture allows cells in vitro to grow in all directions, similar to how they would in vivo. These three-dimensional cultures are usually grown in bioreactors, small capsules in which the cells can grow into spheroids, or 3D cell colonies. Approximately 300 spheroids are usually cultured per bioreactor.
A Hollow fiber bioreactor is a 3 dimensional cell culturing system based on hollow fibers, which are small, semi-permeable capillary membranes arranged in parallel array with a typical molecular weight cut-off (MWCO) range of 10-30 kDa. These hollow fiber membranes are often bundled and housed within tubular polycarbonate shells to create hollow fiber bioreactor cartridges. Within the cartridges, which are also fitted with inlet and outlet ports, are two compartments: the intracapillary (IC) space within the hollow fibers, and the extracapillary (EC) space surrounding the hollow fibers.
Microfluidic cell culture integrates knowledge from biology, biochemistry, engineering, and physics to develop devices and techniques for culturing, maintaining, analyzing, and experimenting with cells at the microscale. It merges microfluidics, a set of technologies used for the manipulation of small fluid volumes within artificially fabricated microsystems, and cell culture, which involves the maintenance and growth of cells in a controlled laboratory environment. Microfluidics has been used for cell biology studies as the dimensions of the microfluidic channels are well suited for the physical scale of cells. For example, eukaryotic cells have linear dimensions between 10-100 μm which falls within the range of microfluidic dimensions. A key component of microfluidic cell culture is being able to mimic the cell microenvironment which includes soluble factors that regulate cell structure, function, behavior, and growth. Another important component for the devices is the ability to produce stable gradients that are present in vivo as these gradients play a significant role in understanding chemotactic, durotactic, and haptotactic effects on cells.
Droplet-based microfluidics manipulate discrete volumes of fluids in immiscible phases with low Reynolds number and laminar flow regimes. Interest in droplet-based microfluidics systems has been growing substantially in past decades. Microdroplets offer the feasibility of handling miniature volumes of fluids conveniently, provide better mixing, encapsulation, sorting, sensing and are suitable for high throughput experiments. Two immiscible phases used for the droplet based systems are referred to as the continuous phase and dispersed phase.

Uwe Marx is a German physician and biotechnologist, and is one of the world's leading researchers in the fields of organ-on-a-chip technology and antibody production. In 1989, he planned to recreate organs like liver, lung, or skin in vitro and to mimic organ functions and interactions outside a living organism. He was initially able to develop a human artificial lymph node model for immunogenicity tests. Since 2007, Marx has been working together with other scientists to reproduce the human organism on a microfluidic chip at a scale of 1:100,000. The aim is to shorten the entire drug development process as well as to reduce animal experiments and drug testing in humans during clinical trials. These microfluidic devices can also be used to test other substances for their safety and efficacy.
Microfluidics refers to the flow of fluid in channels or networks with at least one dimension on the micron scale. In open microfluidics, also referred to as open surface microfluidics or open-space microfluidics, at least one boundary confining the fluid flow of a system is removed, exposing the fluid to air or another interface such as a second fluid.
References
- ↑ Pasirayi, Godfrey; et al. (2011). "Microfluidic Bioreactors for Cell Culturing: A Review" (PDF). Micro and Nanosystems. 3: 137–160. Archived from the original (PDF) on 2017-09-29. Retrieved 2017-09-29.
- ↑ Wong, Pak Kin; et al. (22 October 2004). "Electrokinetic Bioprocessor for Concentrating Cells and Molecules". Analytical Chemistry. 76 (23): 6908–6914. doi:10.1021/ac049479u. PMID 15571340.
| This biotechnology article is a stub. You can help Wikipedia by expanding it. |