Related Research Articles

Oogenesis, ovogenesis, or oögenesis is the differentiation of the ovum into a cell competent to further develop when fertilized. It is developed from the primary oocyte by maturation. Oogenesis is initiated in the embryonic stage.

Eph receptors are a group of receptors that are activated in response to binding with Eph receptor-interacting proteins (Ephrins). Ephs form the largest known subfamily of receptor tyrosine kinases (RTKs). Both Eph receptors and their corresponding ephrin ligands are membrane-bound proteins that require direct cell-cell interactions for Eph receptor activation. Eph/ephrin signaling has been implicated in the regulation of a host of processes critical to embryonic development including axon guidance, formation of tissue boundaries, cell migration, and segmentation. Additionally, Eph/ephrin signaling has been identified to play a critical role in the maintenance of several processes during adulthood including long-term potentiation, angiogenesis, and stem cell differentiation and cancer.

Betaglycan also known as Transforming growth factor beta receptor III (TGFBR3), is a cell-surface chondroitin sulfate / heparan sulfate proteoglycan >300 kDa in molecular weight. Betaglycan binds to various members of the TGF-beta superfamily of ligands via its core protein, and bFGF via its heparan sulfate chains. TGFBR3 is the most widely expressed type of TGF-beta receptor. Its affinity towards all individual isoforms of TGF-beta is similarly high and therefore it plays an important role as a coreceptor mediating the binding of TGF-beta to its other receptors - specifically TGFBR2. The intrinsic kinase activity of this receptor has not yet been described. In regard of TGF-beta signalling it is generally considered a non-signaling receptor or a coreceptor. By binding to various member of the TGF-beta superfamily at the cell surface it acts as a reservoir of TGF-beta.
Stem-cell niche refers to a microenvironment, within the specific anatomic location where stem cells are found, which interacts with stem cells to regulate cell fate. The word 'niche' can be in reference to the in vivo or in vitro stem-cell microenvironment. During embryonic development, various niche factors act on embryonic stem cells to alter gene expression, and induce their proliferation or differentiation for the development of the fetus. Within the human body, stem-cell niches maintain adult stem cells in a quiescent state, but after tissue injury, the surrounding micro-environment actively signals to stem cells to promote either self-renewal or differentiation to form new tissues. Several factors are important to regulate stem-cell characteristics within the niche: cell–cell interactions between stem cells, as well as interactions between stem cells and neighbouring differentiated cells, interactions between stem cells and adhesion molecules, extracellular matrix components, the oxygen tension, growth factors, cytokines, and the physicochemical nature of the environment including the pH, ionic strength and metabolites, like ATP, are also important. The stem cells and niche may induce each other during development and reciprocally signal to maintain each other during adulthood.

Angiopoietin 1 is a type of angiopoietin and is encoded by the gene ANGPT1.
Argos is a secreted protein that is an inhibitor of the epidermal growth factor receptor (EGFR) pathway in Drosophila melanogaster. Argos inhibits the EGFR pathway by sequestering the EGFR ligand Spitz. Argos binds the epidermal growth factor domain of Spitz, preventing interaction between Spitz and EGFR. Argos does not directly interact with EGFR. Argos represents the first example of ligand sequestration as a mechanism of inhibition in the ErbB (EGFR) family.

Transient receptor potential canonical 1 (TRPC1) is a protein that in humans is encoded by the TRPC1 gene.
In early Drosophila development, the first 13 cells pass through mitosis are nuclear divisions (karyokinesis) without cytokinesis, resulting in a multinucleate cell. Pole cells are the cells that form at the polar ends of the Drosophila egg, which begin the adult germ cells. Pole plasm functions to bud the development of pole cells, as well as restore fertilization, even when the cell was previously sterile.

Taste receptor type 2 member 10 is a protein that in humans is encoded by the TAS2R10 gene. The protein is responsible for bitter taste recognition in mammals. It serves as a defense mechanism to prevent consumption of toxic substances which often have a characteristic bitter taste.

Slit homolog 2 protein is a protein that in humans is encoded by the SLIT2 gene.
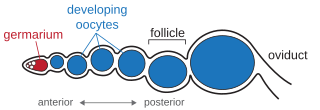
An ovariole is a tubular component of the insect ovary, and the basic unit of egg production. Each ovariole is composed of a germarium at the anterior tip, a set of developing oocytes contained within follicles, and a posterior connection to a common oviduct. While most insects have two ovaries, the number of ovarioles within each ovary varies across insect species. This number may also be variable across individuals within a species, or between the left and right ovaries within an individual.

Notch proteins are a family of type-1 transmembrane proteins that form a core component of the Notch signaling pathway, which is highly conserved in metazoans. The Notch extracellular domain mediates interactions with DSL family ligands, allowing it to participate in juxtacrine signaling. The Notch intracellular domain acts as a transcriptional activator when in complex with CSL family transcription factors. Members of this Type 1 transmembrane protein family share several core structures, including an extracellular domain consisting of multiple epidermal growth factor (EGF)-like repeats and an intracellular domain transcriptional activation domain (TAD). Notch family members operate in a variety of different tissues and play a role in a variety of developmental processes by controlling cell fate decisions. Much of what is known about Notch function comes from studies done in Caenorhabditis elegans (C.elegans) and Drosophila melanogaster. Human homologs have also been identified, but details of Notch function and interactions with its ligands are not well known in this context.
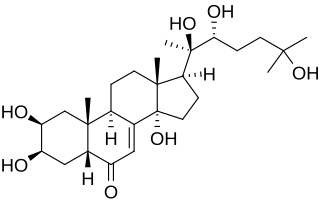
The halloween genes are a set of genes identified in Drosophila melanogaster that influence embryonic development. All of the genes code for cytochrome P450 enzymes in the ecdysteroidogenic pathway (biosynthesis of ecdysone from cholesterol). Ecdysteroids such as 20-hydroxyecdysone and ecdysone influence many of the morphological, physiological, biochemical changes that occur during molting in insects.
Slit-Robo is the name of a cell signaling protein complex with many diverse functions including axon guidance and angiogenesis.

The growth cone is a highly dynamic structure of the developing neuron, changing directionality in response to different secreted and contact-dependent guidance cues; it navigates through the developing nervous system in search of its target. The migration of the growth cone is mediated through the interaction of numerous trophic and tropic factors; netrins, slits, ephrins and semaphorins are four well-studied tropic cues (Fig.1). The growth cone is capable of modifying its sensitivity to these guidance molecules as it migrates to its target; this sensitivity regulation is an important theme seen throughout development.
mir-279 is a short RNA molecule found in Drosophila melanogaster that belongs to a class of molecules known as microRNAs. microRNAs are ~22nt-long non-coding RNAs that post-transcriptionally regulate the expression of genes, often by binding to the 3' untranslated region of mRNA, targeting the transcript for degradation. miR-279 has diverse tissue-specific functions in the fly, influencing developmental processes related to neurogenesis and oogenesis, as well as behavioral processes related to circadian rhythms. The varied roles of mir-279, both in the developing and adult fly, highlight the utility of microRNAs in regulating unique biological processes.
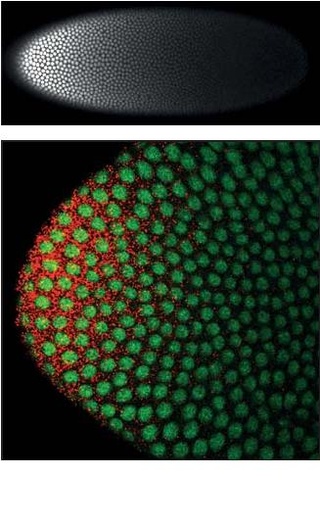
Homeotic protein bicoid is encoded by the bcd maternal effect gene in Drosophilia. Homeotic protein bicoid concentration gradient patterns the anterior-posterior (A-P) axis during Drosophila embryogenesis. Bicoid was the first protein demonstrated to act as a morphogen. Although bicoid is important for the development of Drosophila and other higher dipterans, it is absent from most other insects, where its role is accomplished by other genes.
Oogonial stem cells (OSCs), also known as egg precursor cells or female germline cells, are diploid germline cells with stem cell characteristics: the ability to renew and differentiate into other cell types, different from their tissue of origin. Present in invertebrates and some lower vertebrate species, they have been extensively studied in Caenorhabditis elegans, Drosophila melanogaster. OSCs allow the production of new female reproductive cells (oocytes) by the process of oogenesis during an organism's reproductive life.

Denise Johnson Montell is an American biologist who is the Duggan Professor of Molecular, Cellular, and Developmental Biology at the University of California, Santa Barbara. Her research considers the oogenesis process in Drosophila and border cell migration. She has served as president of the Genetics Society of America and was elected to the National Academy of Sciences in 2021.
The germ cell nest forms in the ovaries during their development. The nest consists of multiple interconnected oogonia formed by incomplete cell division. The interconnected oogonia are surrounded by somatic cells called granulosa cells. Later on in development, the germ cell nests break down through invasion of granulosa cells. The result is individual oogonia surrounded by a single layer of granulosa cells. There is also a comparative germ cell nest structure in the developing spermatogonia, with interconnected intracellular cytoplasmic bridges.
References
- ↑ Ghiglione, Christian; Devergne, Olivier; Georgenthum, Emmanuelle; Carballès, Fabrice; Médioni, Caroline; Cerezo, Delphine; Noselli, Stéphane (2002-12-01). "The Drosophila cytokine receptor Domeless controls border cell migration and epithelial polarization during oogenesis". Development. 129 (23): 5437–5447. doi: 10.1242/dev.00116 . ISSN 1477-9129. PMID 12403714. S2CID 1613810.
- ↑ Montell, D. J.; Rorth, P.; Spradling, A. C. (1992-10-02). "slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP". Cell. 71 (1): 51–62. doi:10.1016/0092-8674(92)90265-e. ISSN 0092-8674. PMID 1394432. S2CID 22360057.
- ↑ Duchek, Peter; Somogyi, Kálmán; Jékely, Gáspár; Beccari, Simone; Rørth, Pernille (2001-10-05). "Guidance of Cell Migration by the Drosophila PDGF/VEGF Receptor". Cell. 107 (1): 17–26. doi: 10.1016/S0092-8674(01)00502-5 . ISSN 0092-8674. PMID 11595182. S2CID 16681564.
- ↑ Bai, Jianwu; Uehara, Yoshihiko; Montell, Denise J. (2000-12-22). "Regulation of Invasive Cell Behavior by Taiman, a Drosophila Protein Related to AIB1, a Steroid Receptor Coactivator Amplified in Breast Cancer". Cell. 103 (7): 1047–1058. doi: 10.1016/S0092-8674(00)00208-7 . ISSN 0092-8674. PMID 11163181. S2CID 13004669.
- ↑ McDonald, Jocelyn A.; Pinheiro, Elaine M.; Kadlec, Lisa; Schupbach, Trudi; Montell, Denise J. (2006-08-01). "Multiple EGFR ligands participate in guiding migrating border cells". Developmental Biology. 296 (1): 94–103. doi: 10.1016/j.ydbio.2006.04.438 . ISSN 0012-1606. PMID 16712835.
- ↑ Bianco, Ambra; Poukkula, Minna; Cliffe, Adam; Mathieu, Juliette; Luque, Carlos M.; Fulga, Tudor A.; Rørth, Pernille (July 2007). "Two distinct modes of guidance signalling during collective migration of border cells". Nature. 448 (7151): 362–365. Bibcode:2007Natur.448..362B. doi:10.1038/nature05965. ISSN 1476-4687. PMID 17637670. S2CID 4369682.
- ↑ Montell, D. J.; Rorth, P.; Spradling, A. C. (1992-10-02). "slow border cells, a locus required for a developmentally regulated cell migration during oogenesis, encodes Drosophila C/EBP". Cell. 71 (1): 51–62. doi:10.1016/0092-8674(92)90265-e. ISSN 0092-8674. PMID 1394432. S2CID 22360057.
- ↑ Cheung, Kevin J.; Ewald, Andrew J. (2016-04-08). "A collective route to metastasis: Seeding by tumor cell clusters". Science. 352 (6282): 167–169. Bibcode:2016Sci...352..167C. doi:10.1126/science.aaf6546. ISSN 1095-9203. PMC 8183671 . PMID 27124449.