
A drop or droplet is a small column of liquid, bounded completely or almost completely by free surfaces. A drop may form when liquid accumulates at the end of a tube or other surface boundary, producing a hanging drop called a pendant drop. Drops may also be formed by the condensation of a vapor or by atomization of a larger mass of solid. Water vapor will condense into droplets depending on the temperature. The temperature at which droplets form is called the dew point.

Dew is water in the form of droplets that appears on thin, exposed objects in the morning or evening due to condensation.
In materials science, the sol–gel process is a method for producing solid materials from small molecules. The method is used for the fabrication of metal oxides, especially the oxides of silicon (Si) and titanium (Ti). The process involves conversion of monomers into a colloidal solution (sol) that acts as the precursor for an integrated network of either discrete particles or network polymers. Typical precursors are metal alkoxides. Sol–gel process is used to produce ceramic nanoparticles.

In fluid mechanics, dewetting is one of the processes that can occur at a solid–liquid, solid–solid or liquid–liquid interface. Generally, dewetting describes the process of retraction of a fluid from a non-wettable surface it was forced to cover. The opposite process—spreading of a liquid on a substrate—is called wetting. The factor determining the spontaneous spreading and dewetting for a drop of liquid placed on a solid substrate with ambient gas, is the so-called spreading coefficient S:

The lotus effect refers to self-cleaning properties that are a result of ultrahydrophobicity as exhibited by the leaves of Nelumbo, the lotus flower. Dirt particles are picked up by water droplets due to the micro- and nanoscopic architecture on the surface, which minimizes the droplet's adhesion to that surface. Ultrahydrophobicity and self-cleaning properties are also found in other plants, such as Tropaeolum (nasturtium), Opuntia, Alchemilla, cane, and also on the wings of certain insects.
The name electrospray is used for an apparatus that employs electricity to disperse a liquid or for the fine aerosol resulting from this process. High voltage is applied to a liquid supplied through an emitter. Ideally the liquid reaching the emitter tip forms a Taylor cone, which emits a liquid jet through its apex. Varicose waves on the surface of the jet lead to the formation of small and highly charged liquid droplets, which are radially dispersed due to Coulomb repulsion.

Sea spray are aerosol particles formed from the ocean, mostly by ejection into Earth's atmosphere by bursting bubbles at the air-sea interface. Sea spray contains both organic matter and inorganic salts that form sea salt aerosol (SSA). SSA has the ability to form cloud condensation nuclei (CCN) and remove anthropogenic aerosol pollutants from the atmosphere. Coarse sea spray has also been found to inhibit the development of lightning in storm clouds.

In chemistry and materials science, ultrahydrophobic surfaces are highly hydrophobic, i.e., extremely difficult to wet. The contact angles of a water droplet on an ultrahydrophobic material exceed 150°. This is also referred to as the lotus effect, after the superhydrophobic leaves of the lotus plant. A droplet striking these kinds of surfaces can fully rebound like an elastic ball. Interactions of bouncing drops can be further reduced using special superhydrophobic surfaces that promote symmetry breaking, pancake bouncing or waterbowl bouncing.

In physics, a "coffee ring" is a pattern left by a puddle of particle-laden liquid after it evaporates. The phenomenon is named for the characteristic ring-like deposit along the perimeter of a spill of coffee. It is also commonly seen after spilling red wine. The mechanism behind the formation of these and similar rings is known as the coffee ring effect or in some instances, the coffee stain effect, or simply ring stain.

Nanofibers are fibers with diameters in the nanometer range. Nanofibers can be generated from different polymers and hence have different physical properties and application potentials. Examples of natural polymers include collagen, cellulose, silk fibroin, keratin, gelatin and polysaccharides such as chitosan and alginate. Examples of synthetic polymers include poly(lactic acid) (PLA), polycaprolactone (PCL), polyurethane (PU), poly(lactic-co-glycolic acid) (PLGA), poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV), and poly(ethylene-co-vinylacetate) (PEVA). Polymer chains are connected via covalent bonds. The diameters of nanofibers depend on the type of polymer used and the method of production. All polymer nanofibers are unique for their large surface area-to-volume ratio, high porosity, appreciable mechanical strength, and flexibility in functionalization compared to their microfiber counterparts.

John Aitken, FRS, FRSE LLD was a Scottish meteorologist, physicist and marine engineer. He was one of the founders of cloud physics and aerosol science, who built the first apparatus to measure the number of dust and fog particles in the atmosphere, a koniscope.
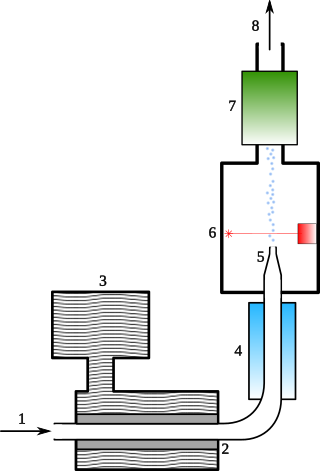
A condensation particle counter or CPC is a particle counter that detects and counts aerosol particles by first enlarging them by using the particles as nucleation centers to create droplets in a supersaturated gas.

Edward Bormashenko is a professor of Materials Science and the Head of the Laboratory of Interface Science of the Ariel University in Israel. He was born in 1962 in Kharkiv, Ukraine and lives in Israel since 1997. He studied in the V. N. Karazin Kharkiv National University. His research is in the polymer science and surface science. He accomplished his PhD in Moscow Institute of Plastics in 1990.
A slippery liquid-infused porous surface (SLIPS), liquid-impregnated surface (LIS), or multi-phase surface consists of two distinct layers. The first is a highly textured or porous substrate with features spaced sufficiently close to stably contain the second layer which is an impregnating liquid that fills in the spaces between the features. The liquid must have a surface energy well-matched to the substrate in order to form a stable film. Slippery surfaces are finding applications in commercial products, anti-fouling surfaces, anti-icing and biofilm-resistant medical devices.

In fluid dynamics, drop impact occurs when a drop of liquid strikes a solid or liquid surface. The resulting outcome depends on the properties of the drop, the surface, and the surrounding fluid, which is most commonly a gas.
Self-cleaning surfaces are a class of materials with the inherent ability to remove any debris or bacteria from their surfaces in a variety of ways. The self-cleaning functionality of these surfaces are commonly inspired by natural phenomena observed in lotus leaves, gecko feet, and water striders to name a few. The majority of self-cleaning surfaces can be placed into three categories:
- superhydrophobic
- superhydrophilic
- photocatalytic.

Liquid marbles are non-stick droplets wrapped by micro- or nano-metrically scaled hydrophobic, colloidal particles ; representing a platform for a diversity of chemical and biological applications. Liquid marbles are also found naturally; aphids convert honeydew droplets into marbles. A variety of non-organic and organic liquids may be converted into liquid marbles. Liquid marbles demonstrate elastic properties and do not coalesce when bounced or pressed lightly. Liquid marbles demonstrate a potential as micro-reactors, micro-containers for growing micro-organisms and cells, micro-fluidics devices, and have even been used in unconventional computing. Liquid marbles remain stable on solid and liquid surfaces. Statics and dynamics of rolling and bouncing of liquid marbles were reported. Liquid marbles coated with poly-disperse and mono-disperse particles have been reported. Liquid marbles are not hermetically coated by solid particles but connected to the gaseous phase. Kinetics of the evaporation of liquid marbles has been investigated.

Droplet cluster is a self-assembled levitating monolayer of microdroplets usually arranged into a hexagonally ordered structure over a locally heated thin layer of water. The droplet cluster is typologically similar to colloidal crystals. The phenomenon was observed for the first time in 2004, and it has been extensively studied after that.

In biochemistry, biomolecular condensates are a class of membrane-less organelles and organelle subdomains, which carry out specialized functions within the cell. Unlike many organelles, biomolecular condensate composition is not controlled by a bounding membrane. Instead, condensates can form and maintain organization through a range of different processes, the most well-known of which is phase separation of proteins, RNA and other biopolymers into either colloidal emulsions, gels, liquid crystals, solid crystals or aggregates within cells.

Passive daytime radiative cooling (PDRC) is a zero-energy building cooling method proposed as a solution to reduce air conditioning, lower urban heat island effect, cool human body temperatures in extreme heat, move toward carbon neutrality and control global warming by enhancing terrestrial heat flow to outer space through the installation of thermally-emissive surfaces on Earth that require zero energy consumption or pollution. In contrast to compression-based cooling systems that are prevalently used, consume substantial amounts of energy, have a net heating effect, require ready access to electricity and often require coolants that are ozone-depleting or have a strong greenhouse effect, application of PDRCs may also increase the efficiency of systems benefiting from a better cooling, such like photovoltaic systems, dew collection techniques, and thermoelectric generators.


















