Nitration is a general class of a chemical process for the introduction of a nitro group into an organic chemical compound. More loosely the term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid, as occurs in the synthesis of nitroglycerin. The difference between the resulting structure of nitro compounds and nitrates is that the nitrogen atom in nitro compounds is directly bonded to a non-oxygen atom, typically carbon or another nitrogen atom, whereas in nitrate esters, also called organic nitrates, the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom.
p-Toluic acid (4-methylbenzoic acid) is a substituted benzoic acid with the formula CH3C6H4CO2H. It is a white solid that is poorly soluble in water but soluble in acetone. A laboratory route to p-toluic acid involves oxidation of p-cymene with nitric acid.
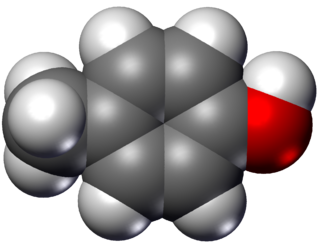
para-Cresol, also 4-methylphenol, is an organic compound with the formula CH3C6H4(OH). It is a colourless solid that is widely used intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of o-cresol and m-cresol.

meta-Cresol, also 3-methylphenol, is an organic compound with the formula CH3C6H4(OH). It is a colourless, viscous liquid that is used as an intermediate in the production of other chemicals. It is a derivative of phenol and is an isomer of p-cresol and o-cresol.
p-Cymene is a naturally occurring aromatic organic compound. It is classified as an alkylbenzene related to a monoterpene. Its structure consists of a benzene ring para-substituted with a methyl group and an isopropyl group. p-Cymene is insoluble in water, but miscible with organic solvents.

The Hofmann–Martius rearrangement in organic chemistry is a rearrangement reaction converting an N-alkylated aniline to the corresponding ortho and / or para aryl-alkylated aniline. The reaction requires heat, and the catalyst is an acid like hydrochloric acid.

Divinylbenzene (DVB) consists of a benzene ring bonded to two vinyl groups. It is related to styrene (vinylbenzene) by the addition of a second vinyl group. It is a colorless liquid manufactured by the thermal dehydrogenation of isomeric diethylbenzenes. Under synthesis conditions, o-divinylbenzene converts to naphthalene and thus is not a component of the usual mixtures of DVB.

Durene, or 1,2,4,5-tetramethylbenzene, is an organic compound with the formula C6H2(CH3)4. It is a colourless solid with a sweet odor. The compound is classified as an alkylbenzene. It is one of three isomers of tetramethylbenzene, the other two being prehnitene (1,2,3,4-tetramethylbenzene) and isodurene (1,2,3,5-tetramethylbenzene. Durene has an unusually high melting point (79.2 °C), reflecting its high molecular symmetry.

(Cymene)ruthenium dichloride dimer is the organometallic compound with the formula [(cymene)RuCl2]2. This red-coloured, diamagnetic solid is a reagent in organometallic chemistry and homogeneous catalysis. The complex is structurally similar to (benzene)ruthenium dichloride dimer.

Organoruthenium chemistry is the chemistry of organometallic compounds containing a carbon to ruthenium chemical bond. Several organoruthenium catalysts are of commercial interest and organoruthenium compounds have been considered for cancer therapy. The chemistry has some stoichiometric similarities with organoiron chemistry, as iron is directly above ruthenium in group 8 of the periodic table. The most important reagents for the introduction of ruthenium are ruthenium(III) chloride and triruthenium dodecacarbonyl.

In chemistry, 1,4-benzoquinonetetracarboxylic acid is an organic compound with formula C
10H
4O
10, or (C6O2)(-(CO)OH)4, which can be viewed as deriving from para-benzoquinoneC
6H
4O
2 through replacement of the four hydrogen atoms by carboxyl functional groups -(CO)OH.
Transalkylation is a chemical reaction involving the transfer of an alkyl group from one organic compound to another. The reaction is used for the transfer of methyl and ethyl groups between benzene rings. This is of particular value in the petrochemical industry to manufacture p-xylene, styrene, and other aromatic compounds. Motivation for using transalkylation reactions is based on a difference in production and demand for benzene, toluene, and xylenes. Transalkylation can convert toluene, which is overproduced, into benzene and xylene, which are under-produced. Zeolites are often used as catalysts in transalkylation reactions.

Prehnitene or 1,2,3,4-tetramethylbenzene, an organic compound with the formula C6H2(CH3)4, is classified as an alkylbenzene. It is one of three isomers of tetramethylbenzene, the other two being isodurine (1,2,3,5-tetramethylbenzene) and durene (1,2,4,5-tetramethylbenzene). It is a relatively easily oxidized benzene derivative, with E1/2 of 2.0 V vs NHE.

Isodurene or 1,2,3,5-tetramethylbenzene, an organic compound with the formula C6H2(CH3)4, is classified as an alkylbenzene. It is one of three isomers of tetramethylbenzene, the other two being prehnitene (1,2,3,4-tetramethylbenzene) and durene (1,2,4,5-tetramethylbenzene).
Pentamethylbenzene is an organic compound with the formula C6H(CH3)5. It is a colourless solid with a sweet odor. The compound is classified as an aromatic hydrocarbon. It is a relatively easily oxidized benzene derivative, with E1/2 of 1.95 V vs NHE.

Half sandwich compounds are organometallic complexes that feature a cyclic polyhapto ligand bound to an MLn center, where L is an unidentate ligand. Thousands of such complexes are known. Well-known examples include cyclobutadieneiron tricarbonyl and (C5H5)TiCl3. Commercially useful examples include (C5H5)Co(CO)2, which is used in the synthesis of substituted pyridines, and methylcyclopentadienyl manganese tricarbonyl, an antiknock agent in petrol.
The cymenes (methylcumenes, isopropyltoluenes) constitute a group of substances of aromatic hydrocarbons, which structure consists of a benzene ring with an isopropyl group (−CH(CH3)2), and a methyl group (−CH3) as a substituent. Through their different arrangement, they form three structural isomers with the molecular formula C10H14. They also belong to the group of C4-benzenes. The best-known isomer is the p-cymene, it occurs in nature and is one of the terpenes.

The tetramethylbenzenes constitute a group of substances of aromatic hydrocarbons, which structure consists of a benzene ring with four methyl groups (–CH3) as a substituent. Through their different arrangement, they form three structural isomers with the molecular formula C10H14. They also belong to the group of C4-benzenes. The best-known isomer is durene.

Pyromellitic dianhydride (PMDA) is an organic compound with the formula C6H2(C2O3)2. It is the double carboxylic acid anhydride that is used in the preparation of polyimide polymers such as Kapton. It is a white solid.

The C4-benzenes are a class of organic aromatic compounds which contain a benzene ring and four other carbon atoms. There are three tetramethylbenzenes, six Dimethylethylbenzenes, three diethylbenzenes, three Isopropylmethylbenzenes, three n-propylmethylbenzenes and four butylbenzenes. The saturated compounds have formula C10H14 and molecular weight 134.22 g/mol. C4-benzenes are found in petroleum. Petrol (gasoline) can contain 5-8% C4-benzenes.











