
In chemistry, an alcohol is a type of organic compound that carries at least one hydroxyl functional group (−OH) bound to a saturated carbon atom. The term alcohol originally referred to the primary alcohol ethanol, which is used as a drug and is the main alcohol present in alcoholic drinks. An important class of alcohols, of which methanol and ethanol are the simplest members, includes all compounds for which the general formula is CnH2n+1OH. Simple monoalcohols that are the subject of this article include primary, secondary and tertiary alcohols.

In chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.

A carboxylic acid is an organic acid that contains a carboxyl group (C(=O)OH) attached to an R-group. The general formula of a carboxylic acid is R−COOH or R−CO2H, with R referring to the alkyl, alkenyl, aryl, or other group. Carboxylic acids occur widely. Important examples include the amino acids and fatty acids. Deprotonation of a carboxylic acid gives a carboxylate anion.

Ethers are a class of organic compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula R–O–R′, where R and R′ represent the alkyl or aryl groups. Ethers can again be classified into two varieties: if the alkyl groups are the same on both sides of the oxygen atom, then it is a simple or symmetrical ether, whereas if they are different, the ethers are called mixed or unsymmetrical ethers. A typical example of the first group is the solvent and anaesthetic diethyl ether, commonly referred to simply as "ether" (CH3–CH2–O–CH2–CH3). Ethers are common in organic chemistry and even more prevalent in biochemistry, as they are common linkages in carbohydrates and lignin.

An ester is a chemical compound derived from an acid in which at least one –OH hydroxyl group is replaced by an –O– alkyl (alkoxy) group, as in the substitution reaction of a carboxylic acid and an alcohol. Glycerides are fatty acid esters of glycerol; they are important in biology, being one of the main classes of lipids and comprising the bulk of animal fats and vegetable oils.

In chemistry, a ketone is a functional group with the structure R2C=O, where R can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond). The simplest ketone is acetone (R = R' = methyl), with the formula CH3C(O)CH3. Many ketones are of great importance in biology and in industry. Examples include many sugars (ketoses), many steroids (e.g., testosterone), and the solvent acetone.
In chemistry, a structural isomer of a compound is another compound whose molecule has the same number of atoms of each element, but with logically distinct bonds between them. The term metamer was formerly used for the same concept.

In chemistry, an aldehyde is an organic compound containing a functional group with the structure −C(H)=O. The functional group itself is known as an aldehyde or formyl group. Aldehydes are common and play important roles in the technology and biological spheres.
In chemistry, a hydration reaction is a chemical reaction in which a substance combines with water. In organic chemistry, water is added to an unsaturated substrate, which is usually an alkene or an alkyne. This type of reaction is employed industrially to produce ethanol, isopropanol, and butan-2-ol.
In chemical nomenclature, the IUPAC nomenclature of organic chemistry is a method of naming organic chemical compounds as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in the Nomenclature of Organic Chemistry. Ideally, every possible organic compound should have a name from which an unambiguous structural formula can be created. There is also an IUPAC nomenclature of inorganic chemistry.
A diol is a chemical compound containing two hydroxyl groups. An aliphatic diol is also called a glycol. This pairing of functional groups is pervasive, and many subcategories have been identified.
1-Hexanol (IUPAC name hexan-1-ol) is an organic alcohol with a six-carbon chain and a condensed structural formula of CH3(CH2)5OH. This colorless liquid is slightly soluble in water, but miscible with diethyl ether and ethanol. Two additional straight chain isomers of 1-hexanol, 2-hexanol and 3-hexanol, exist, both of which differing by the location of the hydroxyl group. Many isomeric alcohols have the formula C6H13OH. It is used in the perfume industry.
Ozonolysis is an organic reaction where the unsaturated bonds of alkenes, alkynes, or azo compounds are cleaved with ozone. Alkenes and alkynes form organic compounds in which the multiple carbon–carbon bond has been replaced by a carbonyl group while azo compounds form nitrosamines. The outcome of the reaction depends on the type of multiple bond being oxidized and the work-up conditions.

tert-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as t-BuOH). It is one of the four isomers of butanol. tert-Butyl alcohol is a colorless solid, which melts near room temperature and has a camphor-like odor. It is miscible with water, ethanol and diethyl ether.
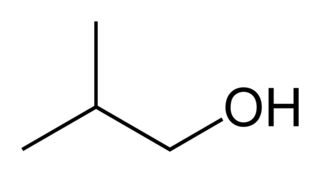
Isobutanol (IUPAC nomenclature: 2-methylpropan-1-ol) is an organic compound with the formula (CH3)2CHCH2OH (sometimes represented as i-BuOH). This colorless, flammable liquid with a characteristic smell is mainly used as a solvent either directly or as its esters. Its isomers, the other butanols, include n-butanol, 2-butanol, and tert-butanol, all of which are important industrially.
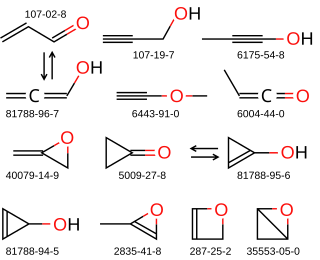
C3H4O is a chemical formula that represents each of several actual and hypothetical compounds that differ in structure, but each consist of three atoms of carbon, four of hydrogen, and one of oxygen. The following compounds are among them:
The molecular formula C3H8O may refer to:

The Mukaiyama taxol total synthesis published by the group of Teruaki Mukaiyama of the Tokyo University of Science between 1997 and 1999 was the 6th successful taxol total synthesis. The total synthesis of Taxol is considered a hallmark in organic synthesis.
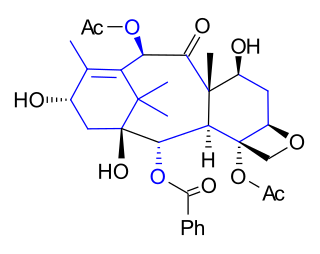
The Takahashi Taxol total synthesis published by Takashi Takahashi in 2006 is one of several methods in taxol total synthesis. The method starts from geraniol and differs from the other 6 published methods that it is a formal synthesis and that it is racemic. A key feature of the published procedure is that several synthetic steps were performed in an automated synthesizer on a scale up to 300 gram and that purification steps were also automated.

o-Cresyl glycidyl ether (ortho-cresyl glycidyl ether, o-CGE) is a liquid aromatic organic chemical compound and chemically a glycidyl ether. It has the formula C10H12O2 and the CAS Registry Number 2210-79-9. It is one of a number of glycidyl ethers available commercially that are used to reduce the viscosity of epoxy resins. These are then further used in coatings, sealants, adhesives and elastomers.













