Sodium laureth sulfate (SLES), an accepted contraction of sodium lauryl ether sulfate (SLES), is an anionic detergent and surfactant found in many personal care products. SLES is an inexpensive and very effective foaming agent. SLES, sodium lauryl sulfate (SLS), ammonium lauryl sulfate (ALS), and sodium pareth sulfate are surfactants that are used in many cosmetic products for their cleaning and emulsifying properties. They behave similarly to soap.
Sulfur trioxide (alternative spelling sulphur trioxide) is the chemical compound with the formula SO3, with a relatively narrow liquid range. In the gaseous form, this species is a significant pollutant, being the primary agent in acid rain.

1,4-Dioxane is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers are rarely encountered.
meta-Chloroperoxybenzoic acid is a peroxycarboxylic acid used widely as an oxidant in organic synthesis. mCPBA is often preferred to other peroxy acids because of its relative ease of handling. The main areas of use are the conversion of ketones to esters, epoxidation of alkenes, conversion of silyl enol ethers to silyl α-hydroxy ketones, oxidation of sulfides to sulfoxides and sulfones, and oxidation of amines to produce amine oxides. mCPBA is a strong oxidizing agent that may cause fire upon contact with flammable material.

Meldrum's acid or 2,2-dimethyl-1,3-dioxane-4,6-dione is an organic compound with formula C
6H
8O
4. Its molecule has a heterocyclic core with four carbon and two oxygen atoms; the formula can also be written as [–O–(C
2)–O–(C=O)–(CH
2)–(C=O)–].
Dioxolane is a heterocyclic acetal with the chemical formula (CH2)2O2CH2. It is related to tetrahydrofuran by interchange of one oxygen for a CH2 group. The corresponding saturated 6-membered C4O2 rings are called dioxanes. The isomeric 1,2-dioxolane (wherein the two oxygen centers are adjacent) is a peroxide. 1,3-Dioxolane is used as a solvent and as a comonomer in polyacetals.
Glyoxal is an organic compound with the chemical formula OCHCHO. It is the smallest dialdehyde. It is a crystalline solid, white at low temperatures and yellow near the melting point (15 °C). The liquid is yellow, and the vapor is green.
Tetrahydropyran (THP) is the organic compound consisting of a saturated six-membered ring containing five carbon atoms and one oxygen atom. It is named by reference to pyran, which contains two double bonds, and may be produced from it by adding four hydrogens. In 2013, its preferred IUPAC name was established as oxane. The compound is a colourless volatile liquid. Derivatives of tetrahydropyran are, however, more common. 2-Tetrahydropyranyl (THP-) ethers derived from the reaction of alcohols and 3,4-dihydropyran are commonly used as protecting groups in organic synthesis. Furthermore, a tetrahydropyran ring system, i.e., five carbon atoms and an oxygen, is the core of pyranose sugars, such as glucose.
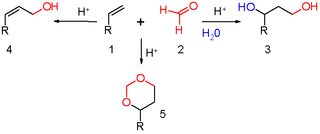
The Prins reaction is an organic reaction consisting of an electrophilic addition of an aldehyde or ketone to an alkene or alkyne followed by capture of a nucleophile. The outcome of the reaction depends on reaction conditions. With water and a protic acid such as sulfuric acid as the reaction medium and formaldehyde the reaction product is a 1,3-diol. When water is absent, the cationic intermediate loses a proton to give an allylic alcohol. With an excess of formaldehyde and a low reaction temperature the reaction product is a dioxane. When water is replaced by acetic acid the corresponding esters are formed.

Lead(IV) acetate or lead tetraacetate is a chemical compound with chemical formula Pb(C2H3O2)4. It is a colorless solid that is soluble in nonpolar organic solvents, indicative that it is not a salt. It is degraded by moisture and is typically stored with additional acetic acid. The compound is used in organic synthesis.

Silver oxalate is commonly employed in experimental petrology to add carbon dioxide to experiments as it will break down to silver (Ag) and carbon dioxide under geologic conditions. It is also a precursor to the production of silver nanoparticles.
It is explosive upon heating around 140 degrees Celsius, shock or friction.

Disodium tetracarbonylferrate is the organoiron compound with the formula Na2[Fe(CO)4]. It is always used as a solvate, e.g. with tetrahydrofuran or dimethoxyethane which bind to the sodium cation. mainly to synthesise aldehydes. This oxygen-sensitive colourless solid is a reagent in organometallic and organic chemical research but is not of commercial value.

Pinacolone (3,3-dimethyl-2-butanone) is an important ketone in organic chemistry. It is a colorless liquid and has a slight peppermint- or camphor- odor. It is a precursor to triazolylpinacolone in the synthesis of the fungicide triadimefon and in synthesis of the herbicide metribuzin. The molecule is an unsymmetrical ketone. The α-methyl group can participate in condensation reactions. The carbonyl group can undergo the usual reactions. It is a Schedule 3 compound under the Chemical Weapons Convention 1993, due to being related to pinacolyl alcohol, which is used in the production of soman. It is also a controlled export in Australia Group member states.

Oxalic anhydride or ethanedioic anhydride, also called oxiranedione, is a hypothetical organic compound with the formula C2O3, which can be viewed as the anhydride of oxalic acid or the two-fold ketone of ethylene oxide. It is an oxide of carbon (an oxocarbon).

Dioxane tetraketone (or 1,4-dioxane-2,3,5,6-tetrone) is an organic compound with the formula C4O6. It is an oxide of carbon (an oxocarbon), which can be viewed as the fourfold ketone of dioxane. It can also be viewed as the cyclic dimer of oxiranedione (C2O3), the hypothetical anhydride of oxalic acid.
Dioxalin is a reaction product of glycerol with oxalic acid at 533 kelvins. Its IUPAC name is 5-(hydroxymethyl)-1,4-dioxane-2,3-dione. Dioxalin readily loses two molecules of carbon dioxide at this high temperature to form allyl alcohol and therefore offers an important method for conversion of glycerol to allyl alcohol.

Lithium chlorate is the inorganic chemical compound with the formula LiClO3.
Like all chlorates, it is an oxidizer and may become unstable and possibly explosive if mixed with organic materials, reactive metal powders, or sulfur.

1,3-Dioxane or m-dioxane is a chemical compound with the molecular formula C4H8O2, the CAS number 505-22-6, EC Number 208-005-1 and RTECS JG8224000.










