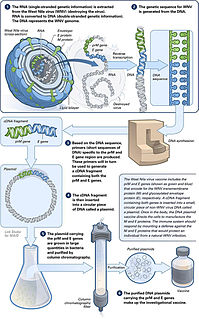
A DNA vaccine is a type of vaccine that transfects a specific antigen-coding DNA sequence into the cells of an organism as a mechanism to induce an immune response.

A poliovirus, the causative agent of polio, is a serotype of the species Enterovirus C, in the family of Picornaviridae. There are three poliovirus serotypes: types 1, 2, and 3.

Simian immunodeficiency virus (SIV) is a species of retrovirus that cause persistent infections in at least 45 species of African non-human primates. Based on analysis of strains found in four species of monkeys from Bioko Island, which was isolated from the mainland by rising sea levels about 11,000 years ago, it has been concluded that SIV has been present in monkeys and apes for at least 32,000 years, and probably much longer.
Polyomaviridae is a family of viruses whose natural hosts are primarily mammals and birds. As of 2020, there are six recognized genera and 117 species, five of which are unassigned to a genus. 14 species are known to infect humans, while others, such as Simian Virus 40, have been identified in humans to a lesser extent. Most of these viruses are very common and typically asymptomatic in most human populations studied. BK virus is associated with nephropathy in renal transplant and non-renal solid organ transplant patients, JC virus with progressive multifocal leukoencephalopathy, and Merkel cell virus with Merkel cell cancer.
SV40 is an abbreviation for simian vacuolating virus 40 or simian virus 40, a polyomavirus that is found in both monkeys and humans. Like other polyomaviruses, SV40 is a DNA virus that has the potential to cause tumors in animals, but most often persists as a latent infection. SV40 has been widely studied as a model eukaryotic virus, leading to many early discoveries in eukaryotic DNA replication and transcription.
A cosmid is a type of hybrid plasmid that contains a Lambda phage cos sequence. They are often used as a cloning vector in genetic engineering. Cosmids can be used to build genomic libraries. They were first described by Collins and Hohn in 1978. Cosmids can contain 37 to 52 kb of DNA, limits based on the normal bacteriophage packaging size. They can replicate as plasmids if they have a suitable origin of replication (ori): for example SV40 ori in mammalian cells, ColE1 ori for double-stranded DNA replication, or f1 ori for single-stranded DNA replication in prokaryotes. They frequently also contain a gene for selection such as antibiotic resistance, so that the transformed cells can be identified by plating on a medium containing the antibiotic. Those cells which did not take up the cosmid would be unable to grow.
Human embryonic kidney 293 cells, also often referred to as HEK 293, HEK-293, 293 cells, or less precisely as HEK cells, are a specific immortalised cell line derived from a spontaneously miscarried or aborted fetus or human embryonic kidney cells grown in tissue culture taken from a female fetus in 1973.
An oncovirus or oncogenic virus is a virus that can cause cancer. This term originated from studies of acutely transforming retroviruses in the 1950–60s, when the term "oncornaviruses" was used to denote their RNA virus origin. With the letters "RNA" removed, it now refers to any virus with a DNA or RNA genome causing cancer and is synonymous with "tumor virus" or "cancer virus". The vast majority of human and animal viruses do not cause cancer, probably because of longstanding co-evolution between the virus and its host. Oncoviruses have been important not only in epidemiology, but also in investigations of cell cycle control mechanisms such as the retinoblastoma protein.

LNCaP cells are a cell line of human cells commonly used in the field of oncology. LNCaP cells are androgen-sensitive human prostate adenocarcinoma cells derived from the left supraclavicular lymph node metastasis from a 50-year-old caucasian male in 1977. They are adherent epithelial cells growing in aggregates and as single cells.
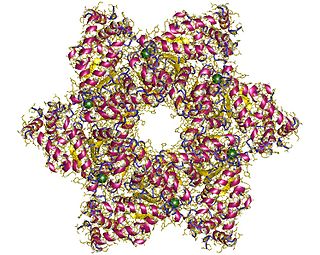
SV40 large T antigen is a hexamer protein that is a dominant-acting oncoprotein derived from the polyomavirus SV40. TAg is capable of inducing malignant transformation of a variety of cell types. The transforming activity of TAg is due in large part to its perturbation of the retinoblastoma (pRb) and p53 tumor suppressor proteins. In addition, TAg binds to several other cellular factors, including the transcriptional co-activators p300 and CBP, which may contribute to its transformation function.

Vero cells are a lineage of cells used in cell cultures. The 'Vero' lineage was isolated from kidney epithelial cells extracted from an African green monkey. The lineage was developed on 27 March 1962, by Yasumura and Kawakita at the Chiba University in Chiba, Japan. The original cell line was named Vero after an abbreviation of verdareno, which means 'green kidney' in Esperanto, while vero itself means 'truth' in Esperanto.
Merkel cell polyomavirus was first described in January 2008 in Pittsburgh, Pennsylvania. It was the first example of a human viral pathogen discovered using unbiased metagenomic next-generation sequencing with a technique called digital transcriptome subtraction. MCV is one of seven currently known human oncoviruses. It is suspected to cause the majority of cases of Merkel cell carcinoma, a rare but aggressive form of skin cancer. Approximately 80% of Merkel cell carcinoma (MCC) tumors have been found to be infected with MCV. MCV appears to be a common—if not universal—infection of older children and adults. It is found in respiratory secretions suggesting that it may be transmitted by a respiratory route. But it also can be found shedding from healthy skin, and in gastrointestinal tract tissues and elsewhere, and so its precise mode of transmission remains unknown. In addition, recent studies suggest that this virus may latently infect the human sera and peripheral blood mononuclear cells.
Epstein–Barr nuclear antigen 1 (EBNA1) is a multifunctional, dimeric viral protein associated with Epstein–Barr virus (EBV). It is the only EBV protein found in all EBV-related malignancies. It is important in establishing and maintaining the altered state that cells take when infected with EBV. EBNA1 has a glycine–alanine repeat sequence that separates the protein into amino- and carboxy-terminal domains. This sequence also seems to stabilize the protein, preventing proteasomal breakdown, as well as impairing antigen processing and MHC class I-restricted antigen presentation. This thereby inhibits the CD8-restricted cytotoxic T cell response against virus-infected cells. EBNA1 is expressed from the Qp promoter during all latency programs. It is the only viral protein expressed in latency program I.
Eugene O. "Gene" Major is a senior investigator at the National Institute of Neurological Disorders and Stroke (NINDS), a part of the United States National Institutes of Health (NIH). Major conducts research into the neurological diseases including progressive multifocal leukoencephalopathy (PML), caused by JC virus and often found in immunosuppressed patients such as those with HIV/AIDS. Major has published over 140 scientific articles and reviews in the peer-reviewed literature and has contributed to Fields Virology, a standard virology textbook.

An immortalised cell line is a population of cells from a multicellular organism which would normally not proliferate indefinitely but, due to mutation, have evaded normal cellular senescence and instead can keep undergoing division. The cells can therefore be grown for prolonged periods in vitro. The mutations required for immortality can occur naturally or be intentionally induced for experimental purposes. Immortal cell lines are a very important tool for research into the biochemistry and cell biology of multicellular organisms. Immortalised cell lines have also found uses in biotechnology.
Mason-Pfizer monkey virus (M-PMV), formerly Simian retrovirus (SRV), is a species of retroviruses that usually infect and cause a fatal immune deficiency in Asian macaques. The ssRNA virus appears sporadically in mammary carcinoma of captive macaques at breeding facilities which expected as the natural host, but the prevalence of this virus in feral macaques remains unknown. M-PMV was transmitted naturally by virus-containing body fluids, via biting, scratching, grooming, and fighting. Cross contaminated instruments or equipment (fomite) can also spread this virus among animals.

WI-38 is a diploid human cell line composed of fibroblasts derived from lung tissue of a 3-month-gestation female fetus. The fetus came from the elective abortion of a Swedish woman in 1963. She was disinterested in the fate of the fetus and its subsequent use in benefitting billions. The cell line was isolated by Leonard Hayflick the same year, and has been used extensively in scientific research, with applications ranging from developing important theories in molecular biology and aging to the production of most human virus vaccines. The uses of this cell line in human virus vaccine production is estimated to have saved the lives of millions of people.

The large tumor antigen is a protein encoded in the genomes of polyomaviruses, which are small double-stranded DNA viruses. LTag is expressed early in the infectious cycle and is essential for viral proliferation. Containing four well-conserved protein domains as well as several intrinsically disordered regions, LTag is a fairly large multifunctional protein; in most polyomaviruses, it ranges from around 600-800 amino acids in length. LTag has two primary functions, both related to replication of the viral genome: it unwinds the virus's DNA to prepare it for replication, and it interacts with proteins in the host cell to dysregulate the cell cycle so that the host's DNA replication machinery can be used to replicate the virus's genome. Some polyomavirus LTag proteins - most notably the well-studied SV40 large tumor antigen from the SV40 virus - are oncoproteins that can induce neoplastic transformation in the host cell.

The small tumor antigen is a protein encoded in the genomes of polyomaviruses, which are small double-stranded DNA viruses. STag is expressed early in the infectious cycle and is usually not essential for viral proliferation, though in most polyomaviruses it does improve replication efficiency. The STag protein is expressed from a gene that overlaps the large tumor antigen (LTag) such that the two proteins share an N-terminal DnaJ-like domain but have distinct C-terminal regions. STag is known to interact with host cell proteins, most notably protein phosphatase 2A (PP2A), and may activate the expression of cellular proteins associated with the cell cycle transition to S phase. In some polyomaviruses - such as the well-studied SV40, which natively infects monkeys - STag is unable to induce neoplastic transformation in the host cell on its own, but its presence may increase the transforming efficiency of LTag. In other polyomaviruses, such as Merkel cell polyomavirus, which causes Merkel cell carcinoma in humans, STag appears to be important for replication and to be an oncoprotein in its own right.
Transient expression, more frequently referred to "transient gene expression", is the temporary expression of genes that are expressed for a short time after nucleic acid, most frequently plasmid DNA encoding an expression cassette, has been introduced into eukaryotic cells with a chemical delivery agent like calcium phosphate (CaPi) or polyethyleneimine (PEI). However, unlike "stable expression," the foreign DNA does not fuse with the host cell DNA, resulting in the inevitable loss of the vector after several cell replication cycles. The majority of transient gene expressions are done with cultivated animal cells. The technique is also used in plant cells; however, the transfer of nucleic acids into these cells requires different methods than those with animal cells. In both plants and animals, transient expression should result in a time-limited use of transferred nucleic acids, since any long-term expression would be called "stable expression."












