
A DNA vaccine is a type of vaccine that transfects a specific antigen-coding DNA sequence into the cells of an organism as a mechanism to induce an immune response.
Immunotherapy or biological therapy is the treatment of disease by activating or suppressing the immune system. Immunotherapies designed to elicit or amplify an immune response are classified as activation immunotherapies, while immunotherapies that reduce or suppress are classified as suppression immunotherapies. Immunotherapy is under preliminary research for its potential to treat various forms of cancer.
A cancer vaccine is a vaccine that either treats existing cancer or prevents development of cancer. Vaccines that treat existing cancer are known as therapeutic cancer vaccines or tumor antigen vaccines. Some of the vaccines are "autologous", being prepared from samples taken from the patient, and are specific to that patient.
Mucin-16(MUC-16) also known as Ovarian cancer-related tumor marker CA125 is a protein that in humans is encoded by the MUC16 gene. MUC-16 is a member of the mucin family glycoproteins. MUC-16 has found application as a tumor marker or biomarker that may be elevated in the blood of some patients with specific types of cancers, most notably ovarian cancer, or other conditions that are benign.

Cancer immunotherapy is the stimulation of the immune system to treat cancer, improving on the immune system's natural ability to fight the disease. It is an application of the fundamental research of cancer immunology and a growing subspecialty of oncology.
Cross-presentation is the ability of certain professional antigen-presenting cells (mostly dendritic cells) to take up, process and present extracellular antigens with MHC class I molecules to CD8 T cells (cytotoxic T cells). Cross-priming, the result of this process, describes the stimulation of naive cytotoxic CD8+ T cells into activated cytotoxic CD8+ T cells. This process is necessary for immunity against most tumors and against viruses that infect dendritic cells and sabotage their presentation of virus antigens. Cross presentation is also required for the induction of cytotoxic immunity by vaccination with protein antigens, for example, tumour vaccination.
Northwest Biotherapeutics is a development-stage American pharmaceutical company headquartered in Maryland that focuses on developing immunotherapies against different types of cancer. It was founded in 1996 by Alton L. Boynton.

Mucin short variant S1, also called polymorphic epithelial mucin (PEM) or epithelial membrane antigen (EMA), is a mucin encoded by the MUC1 gene in humans. Mucin short variant S1 is a glycoprotein with extensive O-linked glycosylation of its extracellular domain. Mucins line the apical surface of epithelial cells in the lungs, stomach, intestines, eyes and several other organs. Mucins protect the body from infection by pathogen binding to oligosaccharides in the extracellular domain, preventing the pathogen from reaching the cell surface. Overexpression of MUC1 is often associated with colon, breast, ovarian, lung and pancreatic cancers. Joyce Taylor-Papadimitriou identified and characterised the antigen during her work with breast and ovarian tumors.

C-type lectin domain family 10 member A (CLEC10A) also designated as CD301 is a protein that in humans is encoded by the CLEC10A gene. CLEC10A is part of the C-type lectin superfamily and binds to N-Acetylgalactosamine (GalNAc). It is mainly expressed on myeloid cells and also on oocytes and very early stages of embryogenesis. CLEC10A is used as a marker of the CD1c+ dendritic cell subgroup, also called cDC2. The actions of CLEC10A are diverse, depending on the ligand and environment.
Tecemotide is a synthetic lipopeptide that is used as antigen in an investigational therapeutic cancer vaccine. The investigational therapeutic cancer vaccine is designed to induce a cellular immune response to cancer cells that express MUC1, a glycoprotein antigen that is widely over-expressed on common cancers such as lung cancer, breast cancer, prostate cancer, and colorectal cancer. The cellular immune response may lead to a rejection of tumor tissue expressing the MUC1 antigen.
Neuvenge, Lapuleucel-T, is a therapeutic cancer vaccine (TCV) in development by Dendreon (DNDN). It uses the "immunotherapy platform approach" first successfully demonstrated on the U.S. Food and Drug Administration (FDA)-approved TCV Provenge. It was first tested on breast cancer patients with tumors expressing HER2/neu, and is now scheduled to be tested on bladder cancer patients.

Joyce Taylor-Papadimitriou FMedSci is a British molecular biologist and geneticist. She is Senior Fellow and Visiting Professor at King's College London specialising in the area of cellular, genetic and proteomic studies on patient breast tumour samples, and works within the Breast Cancer Biology Group. She was the first to identify that the action of interferon type 1 requires the synthesis of effector proteins.

Bavarian Nordic A/S is a fully integrated biotechnology company focused on the development, manufacturing and commercialization of vaccines for infectious diseases and cancer immunotherapies. The company is headquartered in Hellerup, Denmark, with a manufacturing facility in Kvistgård, and an additional site in Hørsholm. The company has a research and development facility in Martinsried, Germany, and offices in Zug, Switzerland, and Morrisville, North Carolina. The company uses viral vectors in its research and development.

Immutep Ltd is a biotechnology company working primarily in the field of cancer immunotherapy using the LAG3 immune control mechanism. The company was originally built on CVac, a therapeutic cancer vaccine. In late 2014 the privately held French immunotherapy company Immutep SA was purchased by Prima Biotech.
Eftilagimod alpha is a large-molecule cancer drug being developed by the clinical-stage biotechnology company Immutep. Efti is a soluble version of the immune checkpoint molecule LAG-3. It is an APC Activator used to increase an immune response to tumors, and is administered by subcutaneous injection. Efti has three intended clinical settings:
Anil Suri is a cancer researcher working in the field of Translational Cancer research at the National Institute of Immunology in New Delhi, India. He is a fellow of the Indian National Academy of Medical Sciences, a fellow of National Academy of Sciences, India, editorial board member of Cancer Research, vice president of the Indian Society for the Study of Reproduction and Fertility (ISSRF) and was an Executive Member of Indian Association of Cancer Research.

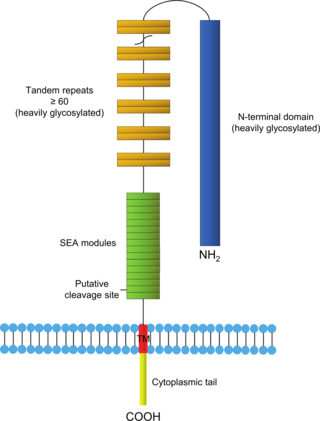
Mucin-1(MUC-1) is a heterodimer transmembrane protein of the mucin family encoded in humans by the MUC1 gene. It is cleaved into two chains: mucin-1 subunit alpha and mucin-1 subunit beta. These subunits differ in size due to proteolytic cleavage of the translated precursor protein in the endoplasmic reticulum. The larger subunit of MUC-1 is characterized by numerous O-glycosylated bonds and a terminal sialic acid, creating a net negative charge on MUC-1. The smaller subunit contains a juxtamembrane region of the extracellular area, a transmembrane domain, and the cytoplasmic tail. The extracellular domain of MUC-1 is composed of 20 identical amino acid tandem repeats (TR). Each tandem repeat contains two serine and three threonine amino acid residues, providing five sites for potential O-glycosylation. MUC-1 protein is estimated to weigh 120 to 225 kDA.
Julianna Lisziewicz is a Hungarian immunologist. Lisziewicz headed many research teams that have discovered and produced immunotheraputic drugs to treat diseases like cancer and chronic infections like HIV/AIDS. Some of these drugs have been successfully used in clinical trials.

An mRNAvaccine is a type of vaccine that uses a copy of a molecule called messenger RNA (mRNA) to produce an immune response. The vaccine delivers molecules of antigen-encoding mRNA into immune cells, which use the designed mRNA as a blueprint to build foreign protein that would normally be produced by a pathogen or by a cancer cell. These protein molecules stimulate an adaptive immune response that teaches the body to identify and destroy the corresponding pathogen or cancer cells. The mRNA is delivered by a co-formulation of the RNA encapsulated in lipid nanoparticles that protect the RNA strands and help their absorption into the cells.

Uğur Şahin is a German oncologist and immunologist. He is the founder and CEO of BioNTech, which developed one of the major vaccines against COVID-19. His main fields of research are cancer research and immunology.









