
Calreticulin also known as calregulin, CRP55, CaBP3, calsequestrin-like protein, and endoplasmic reticulum resident protein 60 (ERp60) is a protein that in humans is encoded by the CALR gene.

In molecular biology, molecular chaperones are proteins that assist the conformational folding or unfolding and the assembly or disassembly of other macromolecular structures. Chaperones are present when the macromolecules perform their normal biological functions and have correctly completed the processes of folding and/or assembly. The chaperones are concerned primarily with protein folding. The first protein to be called a chaperone assists the assembly of nucleosomes from folded histones and DNA and such assembly chaperones, especially in the nucleus, are concerned with the assembly of folded subunits into oligomeric structures.

MHC class I molecules are one of two primary classes of major histocompatibility complex (MHC) molecules and are found on the cell surface of all nucleated cells in the bodies of vertebrates. They also occur on platelets, but not on red blood cells. Their function is to display peptide fragments of proteins from within the cell to cytotoxic T cells; this will trigger an immediate response from the immune system against a particular non-self antigen displayed with the help of an MHC class I protein. Because MHC class I molecules present peptides derived from cytosolic proteins, the pathway of MHC class I presentation is often called cytosolic or endogenous pathway.

Calnexin (CNX) is a 67kDa integral protein of the endoplasmic reticulum (ER). It consists of a large N-terminal calcium-binding lumenal domain, a single transmembrane helix and a short, acidic cytoplasmic tail.

Hippocalcin is a protein that in humans is encoded by the HPCA gene.

The calcitonin receptor (CT) is a G protein-coupled receptor that binds the peptide hormone calcitonin and is involved in maintenance of calcium homeostasis, particularly with respect to bone formation and metabolism.

The granulocyte colony-stimulating factor receptor (G-CSF-R) also known as CD114 is a protein that in humans is encoded by the CSF3R gene. G-CSF-R is a cell-surface receptor for the granulocyte colony-stimulating factor (G-CSF). The G-CSF receptors belongs to a family of cytokine receptors known as the hematopoietin receptor family. The granulocyte colony-stimulating factor receptor is present on precursor cells in the bone marrow, and, in response to stimulation by G-CSF, initiates cell proliferation and differentiation into mature neutrophilic granulocytes and macrophages.

Protein disulfide-isomerase A3 (PDIA3), also known as glucose-regulated protein, 58-kD (GRP58), is an isomerase enzyme. This protein localizes to the endoplasmic reticulum (ER) and interacts with lectin chaperones calreticulin and calnexin (CNX) to modulate folding of newly synthesized glycoproteins. It is thought that complexes of lectins and this protein mediate protein folding by promoting formation of disulfide bonds in their glycoprotein substrates.
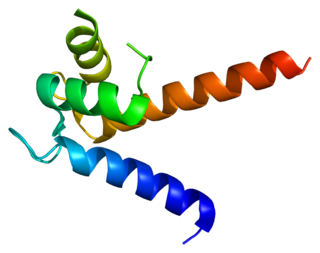
Heparin-binding EGF-like growth factor (HB-EGF) is a member of the EGF family of proteins that in humans is encoded by the HBEGF gene.

Insulin-like growth factor-binding protein 5 is a protein that in humans is encoded by the IGFBP5 gene. An IGFBP5 gene was recently identified as being important for adaptation to varying water salinity in fish.

Peptidyl-prolyl cis-trans isomerase B is an enzyme that is encoded by the PPIB gene. As a member of the peptidyl-prolyl cis-trans isomerase (PPIase) family, this protein catalyzes the cis-trans isomerization of proline imidic peptide bonds, which allows it to regulate protein folding of type I collagen. Generally, PPIases are found in all eubacteria and eukaryotes, as well as in a few archaebacteria, and thus are highly conserved.

Insulin-like growth factor-binding protein 6 (IGFBP-6) is a protein that in humans is encoded by the IGFBP6 gene.

Hematopoietic lineage cell-specific protein is a protein that in humans is encoded by the HCLS1 gene.
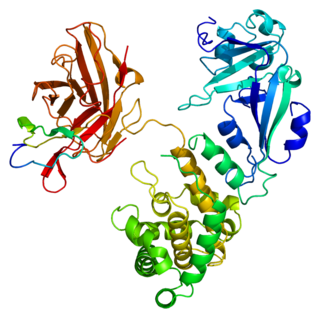
S100 calcium-binding protein P (S100P) is a protein that in humans is encoded by the S100P gene.

SEC14-like protein 2 is a protein that in humans is encoded by the SEC14L2 gene.

N-acylglucosamine 2-epimerase is an enzyme that in humans is encoded by the RENBP gene.

FK506-binding protein 3 is a protein that in humans is encoded by the FKBP3 gene.

Solute carrier family 22 member 11 is a protein that in humans is encoded by the SLC22A11 gene.
Avadhesha Surolia is a Glycobiologist at Indian Institute of Science (IISc), Bangalore. Presently, he is an Honorary Professor at the Molecular Biophysics Unit (MBU), IISc and holds the Bhatnagar fellowship of the Council of Scientific and Industrial Research (CSIR), India. He is known for his work on lectin structure and interactions, orientation and dynamics of cell surface carbohydrate receptors and protein folding, diabetes, anti-malarials and anti-cancer agents based on curcumin, flavonoids, etc. In addition, neuropathic pain, neurodegenerative disorders and the link between immunity and obsessive compulsive disorder are areas of his current interest

The peptide-loading complex (PLC) is a short-lived, multisubunit membrane protein complex that is located in the endoplasmic reticulum (ER). It orchestrates peptide translocation and selection by major histocompatibility complex class I (MHC-I) molecules. Stable peptide-MHC I complexes are released to the cell surface to promote T-cell response against malignant or infected cells. In turn, T-cells recognize the activated peptides, which could be immunogenic or non-immunogenic.



















