Related Research Articles

An artificial cardiac pacemaker, commonly referred to as simply a pacemaker, is an implanted medical device that generates electrical pulses delivered by electrodes to one or more of the chambers of the heart. Each pulse causes the targeted chamber(s) to contract and pump blood, thus regulating the function of the electrical conduction system of the heart.

Cardioversion is a medical procedure by which an abnormally fast heart rate (tachycardia) or other cardiac arrhythmia is converted to a normal rhythm using electricity or drugs. Synchronized electrical cardioversion uses a therapeutic dose of electric current to the heart at a specific moment in the cardiac cycle, restoring the activity of the electrical conduction system of the heart. Pharmacologic cardioversion, also called chemical cardioversion, uses antiarrhythmia medication instead of an electrical shock.

Defibrillation is a treatment for life-threatening cardiac arrhythmias, specifically ventricular fibrillation (V-Fib) and non-perfusing ventricular tachycardia (V-Tach). A defibrillator delivers a dose of electric current to the heart. Although not fully understood, this process depolarizes a large amount of the heart muscle, ending the arrhythmia. Subsequently, the body's natural pacemaker in the sinoatrial node of the heart is able to re-establish normal sinus rhythm. A heart which is in asystole (flatline) cannot be restarted by a defibrillator; it would be treated only by cardiopulmonary resuscitation (CPR) and medication, and then by cardioversion or defibrillation if it converts into a shockable rhythm.

An implantable cardioverter-defibrillator (ICD) or automated implantable cardioverter defibrillator (AICD) is a device implantable inside the body, able to perform defibrillation, and depending on the type, cardioversion and pacing of the heart. The ICD is the first-line treatment and prophylactic therapy for patients at risk for sudden cardiac death due to ventricular fibrillation and ventricular tachycardia.
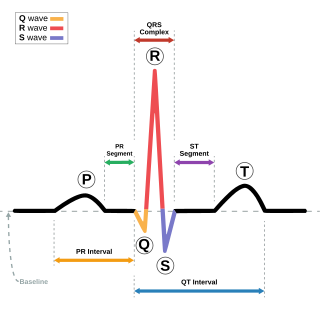
Short QT syndrome (SQT) is a very rare genetic disease of the electrical system of the heart, and is associated with an increased risk of abnormal heart rhythms and sudden cardiac death. The syndrome gets its name from a characteristic feature seen on an electrocardiogram (ECG) – a shortening of the QT interval. It is caused by mutations in genes encoding ion channels that shorten the cardiac action potential, and appears to be inherited in an autosomal dominant pattern. The condition is diagnosed using a 12-lead ECG. Short QT syndrome can be treated using an implantable cardioverter-defibrillator or medications including quinidine. Short QT syndrome was first described in 2000, and the first genetic mutation associated with the condition was identified in 2004.
Ventricular tachycardia is a cardiovascular disorder in which fast heart rate occurs in the ventricles of the heart. Although a few seconds of VT may not result in permanent problems, longer periods are dangerous; and multiple episodes over a short period of time are referred to as an electrical storm. Short periods may occur without symptoms, or present with lightheadedness, palpitations, shortness of breath, chest pain, and decreased level of consciousness. Ventricular tachycardia may lead to coma and persistent vegetative state due to lack of blood and oxygen to the brain. Ventricular tachycardia may result in ventricular fibrillation (VF) and turn into cardiac arrest. This conversion of the VT into VF is called the degeneration of the VT. It is found initially in about 7% of people in cardiac arrest.

Cardiac electrophysiology is a branch of cardiology and basic science focusing on the electrical activities of the heart. The term is usually used in clinical context, to describe studies of such phenomena by invasive (intracardiac) catheter recording of spontaneous activity as well as of cardiac responses to programmed electrical stimulation - clinical cardiac electrophysiology. However, cardiac electrophysiology also encompasses basic research and translational research components. Specialists studying cardiac electrophysiology, either clinically or solely through research, are known as cardiac electrophysiologists.

In cardiology, T wave alternans (TWA) is a periodic beat-to-beat variation in the amplitude or shape of the T wave in an electrocardiogram . TWA was first described in 1908. At that time, only large variations could be detected. Those large TWAs were associated with increased susceptibility to lethal ventricular tachycardias.
St. Jude Medical, Inc. was an American global medical device company headquartered in Little Canada, Minnesota, U.S., a suburb of Saint Paul. The company had more than 20 principal operations and manufacturing facilities worldwide with products sold in more than 100 countries. Its major markets include the United States, Europe, Latin America and Asia-Pacific. The company was named after Jude the Apostle, the patron saint of lost causes.
Clinical cardiac electrophysiology, is a branch of the medical specialty of cardiology concerned with the study and treatment of rhythm disorders of the heart. Cardiologists with expertise in this area are usually referred to as electrophysiologists. Electrophysiologists are trained in the mechanism, function, and performance of the electrical activities of the heart. Electrophysiologists work closely with other cardiologists and cardiac surgeons to assist or guide therapy for heart rhythm disturbances (arrhythmias). They are trained to perform interventional and surgical procedures to treat cardiac arrhythmia.

Transcutaneous pacing (TCP), also called external pacing, is a temporary means of pacing a patient's heart during a medical emergency. It should not be confused with defibrillation using a manual or automatic defibrillator, though some newer defibrillators can do both, and pads and an electrical stimulus to the heart are used in transcutaneous pacing and defibrillation. Transcutaneous pacing is accomplished by delivering pulses of electric current through the patient's chest, which stimulates the heart to contract.
Morton Maimon Mower was an American cardiologist specializing in electrophysiology and the co-inventor of the automatic implantable cardioverter defibrillator. He served in several professional capacities at Sinai Hospital and Cardiac Pacemakers Inc. In 1996, he became the chairman and chief executive officer of Mower Research Associates. He was inducted into the National Inventors Hall of Fame in 2002 for the development of the automatic implantable cardioverter defibrillator with Michel Mirowski in the 1970s. He continued his research in the biomechanical engineering laboratories at Johns Hopkins University.
Alois A. Langer is an American biomedical engineer best known as one of the co-inventors of the Implantable Cardioverter Defibrillator (ICD).

Cardiac resynchronisation therapy is the insertion of electrodes in the left and right ventricles of the heart, as well as on occasion the right atrium, to treat heart failure by coordinating the function of the left and right ventricles via a pacemaker, a small device inserted into the anterior chest wall.
A wearable cardioverter defibrillator (WCD) is a non-invasive, external device for patients at risk of sudden cardiac arrest (SCA). It allows physicians time to assess their patient's arrhythmic risk and see if their ejection fraction improves before determining the next steps in patient care. It is a leased device. A summary of the device, its technology and indications was published in 2017 and reviewed by the EHRA Scientific Documents Committee.
Cardiac contractility modulation is a therapy which is intended for the treatment of patients with moderate to severe heart failure with symptoms despite optimal medical therapy who can benefit from an improvement in cardiac output. The short- and long-term use of this therapy enhances the strength of ventricular contraction and therefore the heart's pumping capacity by modulating (adjusting) the myocardial contractility. This is provided by a pacemaker-like device that applies non-excitatory electrical signals adjusted to and synchronized with the electrical action in the cardiac cycle.
Yaariv Khaykin is a Canadian cardiologist and a clinical researcher in the area of electrophysiology. He is the director of the Newmarket Electrophysiology Research Group at the Southlake Regional Health Centre. He has published research into complex ablation and pioneered cardiac ablation methods.

Cardiac Pacemakers, Inc. (CPI), doing business as Guidant Cardiac Rhythm Management, manufactured implantable cardiac rhythm management devices, such as pacemakers and defibrillators. It sold microprocessor-controlled insulin pumps and equipment to regulate heart rhythm. It developed therapies to treat irregular heartbeat. The company was founded in 1971 and is based in Saint Paul, Minnesota, and is presently a subsidiary of Boston Scientific.

Subcutaneous implantable cardioverter defibrillator, or S-ICD, is an implantable medical device for detecting and terminating ventricular tachycardia and ventricular fibrillation in patients at risk of sudden cardiac arrest. It is a type of implantable cardioverter defibrillator but unlike the transvenous ICD, the S-ICD lead is placed just under the skin, leaving the heart and veins untouched.
Cyborg data mining is the practice of collecting data produced by an implantable device that monitors bodily processes for commercial interests. As an android is a human-like robot, a cyborg, on the other hand, is an organism whose physiological functioning is aided by or dependent upon a mechanical/electronic device that relies on some sort of feedback.
References
- 1 2 3 "Boston Scientific Closes Cameron Health Acquisition". PR Newswire. UBM PLC. 8 June 2012. Retrieved 26 February 2016.
- ↑ "EMBLEM S-ICD System". Boston Scientific. Archived from the original on 26 February 2016. Retrieved 26 February 2016.
- ↑ Bardy GH, Smith WM, Hood MA, Crozier IG, et al. (May 2010). "An Entirely Subcutaneous Implantable Cardioverter–Defibrillator" (PDF). N. Engl. J. Med. 363 (1). New England Journal of Medicine: 36–44. doi:10.1056/NEJMoa0909545. PMID 20463331.
- ↑ Meier, Barry (April 21, 2009). "Study Backs Specialists Implanting Heart Devices". New York Times. Retrieved 19 January 2010.
- ↑ Jaeger, Fredrick J. "Cardiac Arrhythmias". Cleveland Clinic. Retrieved 19 January 2010.
- 1 2 3 Graham-Rowe, Duncan (November 26, 2008). "Internal External Defibrillator". Technology Review. MIT. Retrieved 19 January 2010.
- ↑ Meier, B. (March 13, 2009). "Medtronic Links Device for Heart to 13 Deaths". New York Times. Retrieved 19 January 2010.
- ↑ Burton, Thomas M. (4 February 2010). "Hospitals Dispute Medtronic Data on Wires". Wall Street Journal. pp. D6.
- ↑ Meier, Barry (13 May 2010). "Under-Skin Defibrillators Seen Closer to Reality". The New York Times.
- ↑ Stiles, Steve (December 4, 2008). "ICDs with subcutaneous leads may be just around the corner". The Heart. Retrieved 19 January 2010.
- ↑ O'Riordan, Michael (May 18, 2009). "Subcutaneous ICD system detects and terminates induced ventricular fibrillation". The Heart. Retrieved 19 January 2010.
- ↑ "Stent Company Invests in Defibrillator Maker". Los Angeles Times/Bloomberg News. February 21, 2004. Archived from the original on October 21, 2012. Retrieved 19 January 2010.
- ↑ "Transactions: 50 million raised 05/08". Piper Jaffray. Retrieved 19 January 2010.
- ↑ "Cameron Health Gets $51.5 Million Injection". New York Times. June 17, 2008. Retrieved 19 January 2010.