Related Research Articles

A mitochondrion is a double membrane-bound organelle found in most eukaryotic organisms. Mitochondria generate most of the cell's supply of adenosine triphosphate (ATP), used as a source of chemical energy. Mitochondria were first discovered by Kolliker in the voluntary muscles of insects. A mitochondrion is nicknamed the powerhouse of the cell, first coined by Philip Siekevitz in a 1957 article of the same name.
In cell biology, an organelle is a specialized subunit, usually within a cell, that has a specific function. The name organelle comes from the idea that these structures are parts of cells, as organs are to the body, hence organelle, the suffix -elle being a diminutive. Organelles are either separately enclosed within their own lipid bilayers or are spatially distinct functional units without a surrounding lipid bilayer. Although most organelles are functional units within cells, some functional units that extend outside of cells are often termed organelles, such as cilia, the flagellum and archaellum, and the trichocyst.
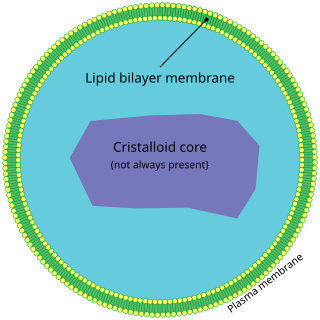
A peroxisome (IPA: [pɛɜˈɹɒksɪˌsoʊm]) is a membrane-bound organelle (formerly known as a microbody), found in the cytoplasm of virtually all eukaryotic cells. Peroxisomes are oxidative organelles. Frequently, molecular oxygen serves as a co-substrate, from which hydrogen peroxide (H2O2) is then formed. Peroxisomes owe their name to hydrogen peroxide generating and scavenging activities. They perform key roles in lipid metabolism and the conversion of reactive oxygen species. Peroxisomes are involved in the catabolism of very long chain fatty acids, branched chain fatty acids, bile acid intermediates (in the liver), D-amino acids, and polyamines, the reduction of reactive oxygen species – specifically hydrogen peroxide – and the biosynthesis of plasmalogens, i.e., ether phospholipids critical for the normal function of mammalian brains and lungs. They also contain approximately 10% of the total activity of two enzymes (Glucose-6-phosphate dehydrogenase and 6-Phosphogluconate dehydrogenase) in the pentose phosphate pathway, which is important for energy metabolism. It is vigorously debated whether peroxisomes are involved in isoprenoid and cholesterol synthesis in animals. Other known peroxisomal functions include the glyoxylate cycle in germinating seeds ("glyoxysomes"), photorespiration in leaves, glycolysis in trypanosomes ("glycosomes"), and methanol and/or amine oxidation and assimilation in some yeasts.
Protein targeting or protein sorting is the biological mechanism by which proteins are transported to their appropriate destinations within or outside the cell. Proteins can be targeted to the inner space of an organelle, different intracellular membranes, the plasma membrane, or to the exterior of the cell via secretion. Information contained in the protein itself directs this delivery process. Correct sorting is crucial for the cell; errors have been linked to multiple disease-states.

Symbiogenesis, endosymbiotic theory, or serial endosymbiotic theory, is the leading evolutionary theory of the origin of eukaryotic cells from prokaryotic organisms. The theory holds that mitochondria, plastids such as chloroplasts, and possibly other organelles of eukaryotic cells are descended from formerly free-living prokaryotes taken one inside the other in endosymbiosis. The idea that chloroplasts were originally independent organisms that merged into a symbiotic relationship with other one-celled organisms dates to the 19th century, espoused by researchers such as Andreas Schimper.

The intermembrane space (IMS) is the space occurring between or involving two or more membranes. In cell biology, it is most commonly described as the region between the inner membrane and the outer membrane of a mitochondrion or a chloroplast. It also refers to the space between the inner and outer nuclear membranes of the nuclear envelope, but is often called the perinuclear space. The IMS of mitochondria plays a crucial role in coordinating a variety of cellular activities, such as regulation of respiration and metabolic functions. Unlike the IMS of the mitochondria, the IMS of the chloroplast does not seem to have any obvious function.

Proteinoplasts are specialized organelles found only in plant cells. Proteinoplasts belong to a broad category of organelles known as plastids. Plastids are specialized double-membrane organelles found in plant cells.Plastids perform a variety of functions such as metabolism of energy, and biological reactions. There are multiple types of plastids recognized including Leucoplasts, Chromoplasts, and Chloroplasts. Plastids are broken up into different categories based on characteristics such as size, function and physical traits. Chromoplasts help to synthesize and store large amounts of carrotenoids. Chloroplasts are photosynthesizing structures that help to make light energy for the plant. Leucoplasts are a colorless type of plastid which means that no photosynthesis occurs here. The colorless pegmentation of the leucoplast is due to not containing the structural components of thylakoids unlike what is found in chloroplasts and chromoplasts that gives them their pigmentation. From leucoplasts stems the subtype, proteinoplasts, which contain proteins for storage. They contain crystalline bodies of protein and can be the sites of enzyme activity involving those proteins. Proteinoplasts are found in many seeds, such as brazil nuts, peanuts and pulses. Although all plastids contain high concentrations of protein, proteinoplasts were identified in the 1960s and 1970s as having large protein inclusions that are visible with both light microscopes and electron microscopes. Other subtypes of Leucoplasts include amyloplast, and elaioplasts. Amyloplasts help to store and synthesize starch molecules found in plants, while elaioplasts synthesize and store lipids in plant cells.
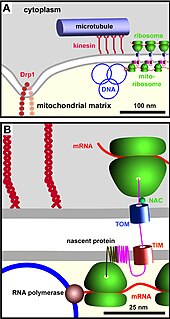
The TIM/TOM complex is a protein complex in cellular biochemistry which translocates proteins produced from nuclear DNA through the mitochondrial membrane for use in oxidative phosphorylation. In enzymology, the complex is described as an mitochondrial protein-transporting ATPase, or more systematically ATP phosphohydrolase , as the TIM part requires ATP hydrolysis to work.

Mitochondrial membrane transport proteins, also known as mitochondrial carrier proteins, are proteins which exist in the membranes of mitochondria. They serve to transport molecules and other factors, such as ions, into or out of the organelles. Mitochondria contain both an inner and outer membrane, separated by the inter-membrane space, or inner boundary membrane. The outer membrane is porous, whereas the inner membrane restricts the movement of all molecules. The two membranes also vary in membrane potential and pH. These factors play a role in the function of mitochondrial membrane transport proteins. There are 53 discovered human mitochondrial membrane transporters, with many others that are known to still need discovered.

Adenine nucleotide translocator (ANT), also known as the ADP/ATP translocase (ANT), ADP/ATP carrier protein (AAC) or mitochondrial ADP/ATP carrier, exchanges free ATP with free ADP across the inner mitochondrial membrane. ANT is the most abundant protein in the inner mitochondrial membrane and belongs to mitochondrial carrier family.
Translocase is a general term for a protein that assists in moving another molecule, usually across a cell membrane. These enzymes catalyze the movement of ions or molecules across membranes or their separation within membranes. The reaction is designated as a transfer from “side 1” to “side 2” because the designations “in” and “out”, which had previously been used, can be ambiguous. Translocases are the most common secretion system in Gram positive bacteria.

Mitochondrial import inner membrane translocase subunit Tim8 A, also known as Deafness-dystonia peptide or protein is an enzyme that in humans is encoded by the TIMM8A gene. This translocase has similarity to yeast mitochondrial proteins that are involved in the import of metabolite transporters from the cytoplasm into the mitochondrial inner membrane. The gene is mutated in Deafness-dystonia syndrome and it is postulated that MTS/DFN-1 is a mitochondrial disease caused by a defective mitochondrial protein import system.

Mitochondrial import receptor subunit TOM20 homolog is a protein that in humans is encoded by the TOMM20 gene.

Mitochondrial import inner membrane translocase subunit Tim13 is an enzyme that in humans is encoded by the TIMM13 gene.

The translocase of the outer membrane (TOM) is a complex of proteins found in the outer mitochondrial membrane of the mitochondria. It allows movement of proteins through this barrier and into the intermembrane space of the mitochondrion. Most of the proteins needed for mitochondrial function are encoded by the nucleus of the cell. The outer membrane of the mitochondrion is impermeable to large molecules greater than 5000 Daltons. The TOM works in conjunction with the translocase of the inner membrane (TIM) to translocate proteins into the mitochondrion. Many of the proteins in the TOM complex, such as TOMM22, were first identified in Neurospora crassa and Saccharomyces cerevisiae.

ADP/ATP translocase 4 (ANT4) is an enzyme that in humans is encoded by the SLC25A31 gene on chromosome 4. This enzyme inhibits apoptosis by catalyzing ADP/ATP exchange across the mitochondrial membranes and regulating membrane potential. In particular, ANT4 is essential to spermatogenesis, as it imports ATP into sperm mitochondria to support their development and survival. Outside this role, the SLC25AC31 gene has not been implicated in any human disease.
The translocase of the inner membrane (TIM) is a complex of proteins found in the inner mitochondrial membrane of the mitochondria. Components of the TIM complex facilitate the translocation of proteins across the inner membrane and into the mitochondrial matrix. They also facilitate the insertion of proteins into the inner mitochondrial membrane, where they must reside in order to function, these mainly include members of the mitochondrial carrier family of proteins.
Mitochondrial biogenesis is the process by which cells increase mitochondrial mass. It was first described by John Holloszy in the 1960s, when it was discovered that physical endurance training induced higher mitochondrial content levels, leading to greater glucose uptake by muscles. Mitochondrial biogenesis is activated by numerous different signals during times of cellular stress or in response to environmental stimuli, such as aerobic exercise.
Mitochondrial processing peptidase is an enzyme complex found in mitochondria which cleaves signal sequences from mitochondrial proteins. In humans this complex is composed of two subunits encoded by the genes PMPCA, and PMPCB. The enzyme is also known as. This enzyme catalyses the following chemical reaction

Jean Vance is a British-Canadian biochemist. She is known for her pioneering work on subcellular organelles and for her discovery of a connection between the endoplasmic reticulum and the mitochondrial membrane. She is a Professor of Medicine at the University of Alberta, Canada and a Fellow of the Royal Society of Canada.
References
- 1 2 3 4 5 6 7 Sedwick, Caitlin (2013-04-29). "Carla Koehler: Small TIMs are a big deal". Journal of Cell Biology. 201 (3): 358–359. doi:10.1083/jcb.2013pi. ISSN 0021-9525. PMC 3639390 .
- 1 2 "Carla Koehler* – UCLA Graduate Programs in Bioscience (GPB)" . Retrieved 2021-05-29.
- 1 2 "Scientific & Medical Advisory Board | UMDF" . Retrieved 2021-05-29.
- ↑ "University of Glasgow - Research Institutes - Institute of Molecular, Cell and Systems Biology - Podcasts". www.gla.ac.uk. Retrieved 2021-05-29.
- ↑ "Carla Koehler". Arnold and Mabel Beckman Foundation. Retrieved 2021-05-29.
- ↑ "Awards & Honors - U Magazine - UCLA Health - Los Angeles, CA". www.uclahealth.org. Retrieved 2021-05-29.
- ↑ "2020-2021 Herbert Newby McCoy Award | UCLA Chemistry and Biochemistry". www.chemistry.ucla.edu. Retrieved 2021-05-29.