
A pseudopod or pseudopodium is a temporary arm-like projection of a eukaryotic cell membrane that is emerged in the direction of movement. Filled with cytoplasm, pseudopodia primarily consist of actin filaments and may also contain microtubules and intermediate filaments. Pseudopods are used for motility and ingestion. They are often found in amoebas.
Mechanotaxis refers to the directed movement of cell motility via mechanical cues. In response to fluidic shear stress, for example, cells have been shown to migrate in the direction of the fluid flow. Mechanotaxis is critical in many normal biological processes in animals, such as gastrulation, inflammation, and repair in response to a wound, as well as in mechanisms of diseases such as tumor metastasis.
Cell migration is a central process in the development and maintenance of multicellular organisms. Tissue formation during embryonic development, wound healing and immune responses all require the orchestrated movement of cells in particular directions to specific locations. Cells often migrate in response to specific external signals, including chemical signals and mechanical signals. Errors during this process have serious consequences, including intellectual disability, vascular disease, tumor formation and metastasis. An understanding of the mechanism by which cells migrate may lead to the development of novel therapeutic strategies for controlling, for example, invasive tumour cells.
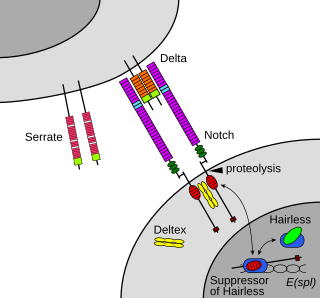
In biology, juxtacrine signalling is a type of cell–cell or cell–extracellular matrix signalling in multicellular organisms that requires close contact. In this type of signalling, a ligand on one surface binds to a receptor on another adjacent surface. Hence, this stands in contrast to releasing a signaling molecule by diffusion into extracellular space, the use of long-range conduits like membrane nanotubes and cytonemes or the use of extracellular vesicles like exosomes or microvesicles. There are three types of juxtacrine signaling:
- A membrane-bound ligand and a membrane protein of two adjacent cells interact.
- A communicating junction links the intracellular compartments of two adjacent cells, allowing transit of relatively small molecules.
- An extracellular matrix glycoprotein and a membrane protein interact.

Eph receptors are a group of receptors that are activated in response to binding with Eph receptor-interacting proteins (Ephrins). Ephs form the largest known subfamily of receptor tyrosine kinases (RTKs). Both Eph receptors and their corresponding ephrin ligands are membrane-bound proteins that require direct cell-cell interactions for Eph receptor activation. Eph/ephrin signaling has been implicated in the regulation of a host of processes critical to embryonic development including axon guidance, formation of tissue boundaries, cell migration, and segmentation. Additionally, Eph/ephrin signaling has been identified to play a critical role in the maintenance of several processes during adulthood including long-term potentiation, angiogenesis, and stem cell differentiation and cancer.
The Rho family of GTPases is a family of small signaling G proteins, and is a subfamily of the Ras superfamily. The members of the Rho GTPase family have been shown to regulate many aspects of intracellular actin dynamics, and are found in all eukaryotic kingdoms, including yeasts and some plants. Three members of the family have been studied in detail: Cdc42, Rac1, and RhoA. All G proteins are "molecular switches", and Rho proteins play a role in organelle development, cytoskeletal dynamics, cell movement, and other common cellular functions.

Ephrins are a family of proteins that serve as the ligands of the Eph receptor. Eph receptors in turn compose the largest known subfamily of receptor protein-tyrosine kinases (RTKs).
G12/G13 alpha subunits are alpha subunits of heterotrimeric G proteins that link cell surface G protein-coupled receptors primarily to guanine nucleotide exchange factors for the Rho small GTPases to regulate the actin cytoskeleton. Together, these two proteins comprise one of the four classes of G protein alpha subunits. G protein alpha subunits bind to guanine nucleotides and function in a regulatory cycle, and are active when bound to GTP but inactive and associated with the G beta-gamma complex when bound to GDP. G12/G13 are not targets of pertussis toxin or cholera toxin, as are other classes of G protein alpha subunits.

Cell division control protein 42 homolog is a protein that in humans is encoded by the CDC42 gene. Cdc42 is involved in regulation of the cell cycle. It was originally identified in S. cerevisiae (yeast) as a mediator of cell division, and is now known to influence a variety of signaling events and cellular processes in a variety of organisms from yeast to mammals.

Transforming protein RhoA, also known as Ras homolog family member A (RhoA), is a small GTPase protein in the Rho family of GTPases that in humans is encoded by the RHOA gene. While the effects of RhoA activity are not all well known, it is primarily associated with cytoskeleton regulation, mostly actin stress fibers formation and actomyosin contractility. It acts upon several effectors. Among them, ROCK1 and DIAPH1 are the best described. RhoA, and the other Rho GTPases, are part of a larger family of related proteins known as the Ras superfamily, a family of proteins involved in the regulation and timing of cell division. RhoA is one of the oldest Rho GTPases, with homologues present in the genomes since 1.5 billion years. As a consequence, RhoA is somehow involved in many cellular processes which emerged throughout evolution. RhoA specifically is regarded as a prominent regulatory factor in other functions such as the regulation of cytoskeletal dynamics, transcription, cell cycle progression and cell transformation.

EPH receptor A2 is a protein that in humans is encoded by the EPHA2 gene.

RhoC is a small signaling G protein, and is a member of the Rac subfamily of the family Rho family of GTPases. It is encoded by the gene RHOC.

Ephrin A1 is a protein that in humans is encoded by the EFNA1 gene.

Ephrin type-A receptor 6 is a protein that in humans is encoded by the EPHA6 gene.
Cell polarity refers to spatial differences in shape, structure, and function within a cell. Almost all cell types exhibit some form of polarity, which enables them to carry out specialized functions. Classical examples of polarized cells are described below, including epithelial cells with apical-basal polarity, neurons in which signals propagate in one direction from dendrites to axons, and migrating cells. Furthermore, cell polarity is important during many types of asymmetric cell division to set up functional asymmetries between daughter cells.
Ralf Heinrich Adams is a biochemist and cell biologist. He is director at the Max Planck Institute for Molecular Biomedicine and head of the Department of Tissue Morphogenesis in Münster, Germany.

Alan Hall FRS was a British cell biologist and a biology professor at the Sloan-Kettering Institute, where he was chair of the Cell Biology program. Hall was elected a Fellow of the Royal Society in 1999.

Rong Li is the Director of Mechanobiology Institute, a Singapore Research Center of Excellence, at the National University of Singapore. She is a Distinguished Professor at the National University of Singapore's Department of Biological Sciences and Bloomberg Distinguished Professor of Cell Biology and Chemical & Biomolecular Engineering at the Johns Hopkins School of Medicine and Whiting School of Engineering. She previously served as Director of Center for Cell Dynamics in the Johns Hopkins School of Medicine’s Institute for Basic Biomedical Sciences. She is a leader in understanding cellular asymmetry, division and evolution, and specifically, in how eukaryotic cells establish their distinct morphology and organization in order to carry out their specialized functions.

Anne Jacqueline Ridley is professor of Cell Biology and Head of School for Cellular and Molecular Medicine at the University of Bristol. She was previously a professor at King's College London.

Synaptic stabilization is crucial in the developing and adult nervous systems and is considered a result of the late phase of long-term potentiation (LTP). The mechanism involves strengthening and maintaining active synapses through increased expression of cytoskeletal and extracellular matrix elements and postsynaptic scaffold proteins, while pruning less active ones. For example, cell adhesion molecules (CAMs) play a large role in synaptic maintenance and stabilization. Gerald Edelman discovered CAMs and studied their function during development, which showed CAMs are required for cell migration and the formation of the entire nervous system. In the adult nervous system, CAMs play an integral role in synaptic plasticity relating to learning and memory.















