
In physics and chemistry, an equation of state is a thermodynamic equation relating state variables, which describe the state of matter under a given set of physical conditions, such as pressure, volume, temperature, or internal energy. Most modern equations of state are formulated in the Helmholtz free energy. Equations of state are useful in describing the properties of pure substances and mixtures in liquids, gases, and solid states as well as the state of matter in the interior of stars.

Sonoluminescence is the emission of light from imploding bubbles in a liquid when excited by sound.

The van der Waals equation, named for its originator, the Dutch physicist Johannes Diderik van der Waals, is an equation of state that extends the ideal gas law to include the non-zero size of gas molecules and the interactions between them. As a result the equation is able to model the phase change, liquid vapor. It also produces simple analytic expressions for the properties of real substances that shed light on their behavior. One way to write this equation is: where is pressure, is temperature, and is molar volume, is the Avogadro constant, is the volume, and is the number of molecules. In addition is the universal gas constant, is the Boltzmann constant, and and are experimentally determinable, substance-specific constants.

Foams are materials formed by trapping pockets of gas in a liquid or solid.
In fluid dynamics, the pressure coefficient is a dimensionless number which describes the relative pressures throughout a flow field. The pressure coefficient is used in aerodynamics and hydrodynamics. Every point in a fluid flow field has its own unique pressure coefficient, Cp.

The Rayleigh–Taylor instability, or RT instability, is an instability of an interface between two fluids of different densities which occurs when the lighter fluid is pushing the heavier fluid. Examples include the behavior of water suspended above oil in the gravity of Earth, mushroom clouds like those from volcanic eruptions and atmospheric nuclear explosions, supernova explosions in which expanding core gas is accelerated into denser shell gas, instabilities in plasma fusion reactors and inertial confinement fusion.
Choked flow is a compressible flow effect. The parameter that becomes "choked" or "limited" is the fluid velocity.
The Ostwald–Freundlich equation governs boundaries between two phases; specifically, it relates the surface tension of the boundary to its curvature, the ambient temperature, and the vapor pressure or chemical potential in the two phases.

In fluid mechanics, multiphase flow is the simultaneous flow of materials with two or more thermodynamic phases. Virtually all processing technologies from cavitating pumps and turbines to paper-making and the construction of plastics involve some form of multiphase flow. It is also prevalent in many natural phenomena.
The Kelvin equation describes the change in vapour pressure due to a curved liquid–vapor interface, such as the surface of a droplet. The vapor pressure at a convex curved surface is higher than that at a flat surface. The Kelvin equation is dependent upon thermodynamic principles and does not allude to special properties of materials. It is also used for determination of pore size distribution of a porous medium using adsorption porosimetry. The equation is named in honor of William Thomson, also known as Lord Kelvin.

A bubble is a globule of a gas substance in a liquid. In the opposite case, a globule of a liquid in a gas, is called a drop. Due to the Marangoni effect, bubbles may remain intact when they reach the surface of the immersive substance.
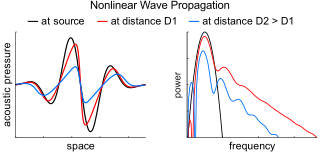
Nonlinear acoustics (NLA) is a branch of physics and acoustics dealing with sound waves of sufficiently large amplitudes. Large amplitudes require using full systems of governing equations of fluid dynamics and elasticity. These equations are generally nonlinear, and their traditional linearization is no longer possible. The solutions of these equations show that, due to the effects of nonlinearity, sound waves are being distorted as they travel.
In fluid thermodynamics, nucleate boiling is a type of boiling that takes place when the surface temperature is hotter than the saturated fluid temperature by a certain amount but where the heat flux is below the critical heat flux. For water, as shown in the graph below, nucleate boiling occurs when the surface temperature is higher than the saturation temperature by between 10 and 30 °C. The critical heat flux is the peak on the curve between nucleate boiling and transition boiling. The heat transfer from surface to liquid is greater than that in film boiling.

A bubble column reactor is a chemical reactor that belongs to the general class of multiphase reactors, which consists of three main categories: trickle bed reactor, fluidized bed reactor, and bubble column reactor. A bubble column reactor is a very simple device consisting of a vertical vessel filled with water with a gas distributor at the inlet. Due to the ease of design and operation, which does not involve moving parts, they are widely used in the chemical, biochemical, petrochemical, and pharmaceutical industries to generate and control gas-liquid chemical reactions.
The vaporizing droplet problem is a challenging issue in fluid dynamics. It is part of many engineering situations involving the transport and computation of sprays: fuel injection, spray painting, aerosol spray, flashing releases… In most of these engineering situations there is a relative motion between the droplet and the surrounding gas. The gas flow over the droplet has many features of the gas flow over a rigid sphere: pressure gradient, viscous boundary layer, wake. In addition to these common flow features one can also mention the internal liquid circulation phenomenon driven by surface-shear forces and the boundary layer blowing effect.
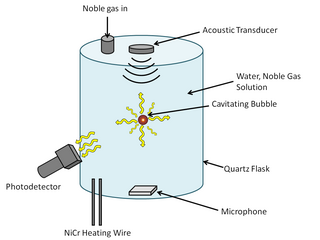
Sonoluminescence is a phenomenon that occurs when a small gas bubble is acoustically suspended and periodically driven in a liquid solution at ultrasonic frequencies, resulting in bubble collapse, cavitation, and light emission. The thermal energy that is released from the bubble collapse is so great that it can cause weak light emission. The mechanism of the light emission remains uncertain, but some of the current theories, which are categorized under either thermal or electrical processes, are Bremsstrahlung radiation, argon rectification hypothesis, and hot spot. Some researchers are beginning to favor thermal process explanations as temperature differences have consistently been observed with different methods of spectral analysis. In order to understand the light emission mechanism, it is important to know what is happening in the bubble's interior and at the bubble's surface.
In fluid mechanics, the Tait equation is an equation of state, used to relate liquid density to hydrostatic pressure. The equation was originally published by Peter Guthrie Tait in 1888 in the form

In fluid mechanics, the Rayleigh–Plesset equation or Besant–Rayleigh–Plesset equation is a nonlinear ordinary differential equation which governs the dynamics of a spherical bubble in an infinite body of incompressible fluid. Its general form is usually written as
Blade element momentum theory is a theory that combines both blade element theory and momentum theory. It is used to calculate the local forces on a propeller or wind-turbine blade. Blade element theory is combined with momentum theory to alleviate some of the difficulties in calculating the induced velocities at the rotor.
Bjerknes forces are translational forces on bubbles in a sound wave. The phenomenon is a type of acoustic radiation force. Primary Bjerknes forces are caused by an external sound field; secondary Bjerknes forces are attractive or repulsive forces between pairs of bubbles in the same sound field caused by the pressure field generated by each bubble volume's oscillations. They were first described by Vilhelm Bjerknes in his 1906 Fields of Force.
















