
A vaccine trial is a clinical trial that aims at establishing the safety and efficacy of a vaccine prior to it being licensed.
In clinical trials, a surrogate endpoint is a measure of effect of a specific treatment that may correlate with a real clinical endpoint but does not necessarily have a guaranteed relationship. The National Institutes of Health (USA) defines surrogate endpoint as "a biomarker intended to substitute for a clinical endpoint".

Cetuximab, sold under the brand name Erbitux, is an epidermal growth factor receptor (EGFR) inhibitor medication used for the treatment of metastatic colorectal cancer and head and neck cancer. Cetuximab is a chimeric (mouse/human) monoclonal antibody given by intravenous infusion.

Drug development is the process of bringing a new pharmaceutical drug to the market once a lead compound has been identified through the process of drug discovery. It includes preclinical research on microorganisms and animals, filing for regulatory status, such as via the United States Food and Drug Administration for an investigational new drug to initiate clinical trials on humans, and may include the step of obtaining regulatory approval with a new drug application to market the drug. The entire process—from concept through preclinical testing in the laboratory to clinical trial development, including Phase I–III trials—to approved vaccine or drug typically takes more than a decade.
Panitumumab, sold under the brand name Vectibix, is a fully human monoclonal antibody specific to the epidermal growth factor receptor.
GeoVax is a clinical-stage biotechnology company which develops vaccines. GeoVax's development platform uses Modified Vaccinia Ankara (MVA) vector technology, with improvements to antigen design and manufacturing capabilities. GeoVax uses recombinant DNA or recombinant viruses to produce virus-like particles (VLPs) in the person being vaccinated.
Leronlimab is a humanized monoclonal antibody targeted against the CCR5 receptor found on T lymphocytes of the human immune system. It is being investigated as a potential therapy in the treatment of COVID-19, triple negative breast cancer, and HIV infection. The United States Food and Drug Administration has designated PRO 140 for fast-track approval. In February 2008, the drug entered Phase 2 clinical trials and a phase 3 trial was begun in 2015. In February 2018, Cytodyn Inc reported that the primary endpoint had been achieved in the PRO 140 pivotal combination therapy trial in HIV infection. In 2020 CytoDyn submitted a fast-track biologics license application for treatment of CCR5-tropic HIV-1 Infection.

Genta Incorporated was a biopharmaceutical company started in La Jolla, California, which discovered and developed innovative drugs for the treatment of patients with cancer. Founded in 1989 by a highly skilled entrepreneur, the company focused on a novel technology known as antisense, which targets gene products that are associated with the onset and progression of serious diseases. At that time, only Ionis Pharmaceuticals, Inc. was conducting significant research with this technology. Antisense is a short span of oligonucleotides – modified DNA structures ranging from about 12-24 bases that selectively bind to specific RNA. The intent is to block expression of an aberrant protein that contributes to the disease of interest. Genta in-licensed three different antisense molecules that blocked Bcl-2, a fibroblast growth factor (FGF), and the gene c-myb, respectively.
Teprotumumab, sold under the brand name Tepezza, is a medication used to treat adults with thyroid eye disease, a rare condition where the muscles and fatty tissues behind the eye become inflamed, causing the eyes to bulge outwards.
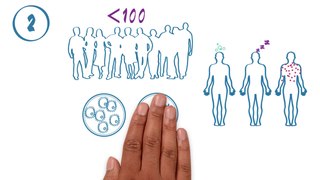
The phases of clinical research are the stages in which scientists conduct experiments with a health intervention to obtain sufficient evidence for a process considered effective as a medical treatment. For drug development, the clinical phases start with testing for drug safety in a few human subjects, then expand to many study participants to determine if the treatment is effective. Clinical research is conducted on drug candidates, vaccine candidates, new medical devices, and new diagnostic assays.
Nivolumab, sold under the brand name Opdivo, is an anti-cancer medication used to treat a number of types of cancer. This includes melanoma, lung cancer, malignant pleural mesothelioma, renal cell carcinoma, Hodgkin lymphoma, head and neck cancer, urothelial carcinoma, colon cancer, esophageal squamous cell carcinoma, liver cancer, gastric cancer, and esophageal or gastroesophageal junction cancer. It is administered intravenously.

Pembrolizumab, sold under the brand name Keytruda, is a humanized antibody used in cancer immunotherapy that treats melanoma, lung cancer, head and neck cancer, Hodgkin lymphoma, stomach cancer, cervical cancer, and certain types of breast cancer. It is administered by slow intravenous injection.
The United States Food and Drug Administration (FDA) initiated the FDA Accelerated Approval Program in 1992 to allow faster approval of drugs for serious conditions that fill an unmet medical need. The faster approval relies on use of surrogate endpoints. Drug approval typically requires clinical trials with endpoints that demonstrate a clinical benefit, such as increased survival for cancer patients. Drugs with accelerated approval can initially be tested in clinical trials that use a surrogate endpoint, or something that is thought to predict clinical benefit. Surrogate endpoints typically require less time, and in the case of a cancer patient, it is much faster to measure a reduction in tumor size, for example, than overall patient survival.

Durvalumab, sold under the brand name Imfinzi, is an FDA-approved immunotherapy for cancer, developed by Medimmune/AstraZeneca. It is a human immunoglobulin G1 kappa (IgG1κ) monoclonal antibody that blocks the interaction of programmed cell death ligand 1 (PD-L1) with the PD-1 (CD279).
Rindopepimut (CDX-110) is an injectable peptide cancer vaccine which targets a mutant protein called EGFRvIII present in about 25% to 30% of glioblastoma cases.
Tonix Pharmaceuticals is a pharmaceutical company based in Chatham, New Jersey that focuses on repurposed drugs for central nervous system conditions and as of 2020 was also pursuing a vaccine for COVID-19 and a biodefense project.
Valneva SE is a French biotech company headquartered in Saint-Herblain, France, developing and commercializing vaccines for infectious diseases. It has manufacturing sites in Livingston, Scotland; Solna, Sweden, and Vienna, Austria; with other offices in France, Canada and the United States.

COVID-19 drug development is the research process to develop preventative therapeutic prescription drugs that would alleviate the severity of coronavirus disease 2019 (COVID-19). From early 2020 through 2021, several hundred drug companies, biotechnology firms, university research groups, and health organizations were developing therapeutic candidates for COVID-19 disease in various stages of preclinical or clinical research, with 419 potential COVID-19 drugs in clinical trials, as of April 2021.
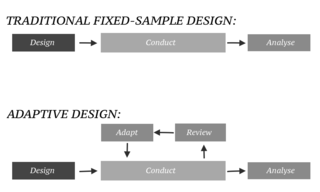
In an adaptive design of a clinical trial, the parameters and conduct of the trial for a candidate drug or vaccine may be changed based on an interim analysis. Adaptive design typically involves advanced statistics to interpret a clinical trial endpoint. This is in contrast to traditional single-arm clinical trials or randomized clinical trials (RCTs) that are static in their protocol and do not modify any parameters until the trial is completed. The adaptation process takes place at certain points in the trial, prescribed in the trial protocol. Importantly, this trial protocol is set before the trial begins with the adaptation schedule and processes specified. Adaptions may include modifications to: dosage, sample size, drug undergoing trial, patient selection criteria and/or "cocktail" mix. The PANDA provides not only a summary of different adaptive designs, but also comprehensive information on adaptive design planning, conduct, analysis and reporting.

The MVC COVID-19 vaccine, designated MVC-COV1901 and also known as the Medigen COVID-19 vaccine, is a protein subunit COVID-19 vaccine developed by Medigen Vaccine Biologics Corporation in Taiwan, American company Dynavax Technologies, and the U.S. National Institutes of Health.









