History
Apoptosis
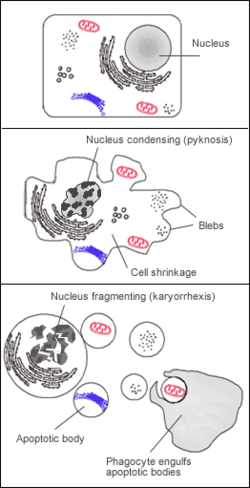
Apoptosis, or programmed cell death, was discovered in 1842 by Carl Vogt and was initially believed to be irreversible. [2] Once a cell exhibited signs of apoptosis, the cell was doomed. Apoptosis is triggered by external or internal signals, such as developmental cues or cellular damage, which activate cellular pathways leading to apoptosis. Cells at risk of cell death display shrinkage and membrane blebbing. [3] Once the pathway to cellular death is initiated, an enzyme known as caspase, a proteolytic cysteine, is activated. Caspase breaks down proteins and DNA in the cells, preparing the cell for removal by phagocytes. [4] Should apoptosis be restricted or prevented, uncontrolled cell division and tumor growth may occur. [5] Apoptosis has a significant role in maintaining tissue homeostasis by eliminating damaged cells and preventing tumor formation. Cells that are no longer needed or damaged are targeted for apoptosis, aiding in the regulation of normal conditions and body functioning. [ citation needed ]
Anastasis
Apoptosis was once considered irreversible and unavoidable before the recent discovery of the process of anastasis. It is a rapid process with many initiating factors that were once believed to be permanent. [6] Anastasis, meaning rising to life, was a term coined by siblings Ho Man Tang and Ho Lam Tang following their discovery at the University of Hong Kong in 2007. [7] [8] The Tang siblings executed their experiment by exposing breast cancer cells to various toxic chemicals and waiting for signs of apoptosis. After the cells displayed these characteristics, they then washed the cells with fresh medium and allowed them to incubate. The cancer cells were induced into apoptosis by treating them with ethanol, and the results showed that survival of these cells was possible. [9] Many cells in the original study survived apoptosis and appeared normal once again following the washing of the cells by fresh medium. Tang's results were not initially well received due to the popular opinion at the time that apoptosis was irreversible. However, their research eventually became more accepted and challenged the traditional understanding of apoptosis as an irreversible process. The discovery of anastasis suggested that cells have the potential to reverse the process of cell death under certain circumstances. [ citation needed ]
Etymology

The word Anastasis comes from the Greek word for resurrection, ανάσταση. [10] The prefix ana- means "upward" or "again", and the root sta- means "to stand", forming a combined meaning of "standing again" or "resurrection". In Christianity, the term anastasis refers to the resurrection of Jesus Christ. The term is used to describe the notion of rising or standing again after a period of death or dormancy. The use of the word anastasis began increasing steadily following the Tang siblings' discovery in 2007. [11]
