
A colloid is a mixture in which one substance consisting of microscopically dispersed insoluble particles is suspended throughout another substance. Some definitions specify that the particles must be dispersed in a liquid, while others extend the definition to include substances like aerosols and gels. The term colloidal suspension refers unambiguously to the overall mixture. A colloid has a dispersed phase and a continuous phase. The dispersed phase particles have a diameter of approximately 1 nanometre to 1 micrometre.
An intermolecular force is the force that mediates interaction between molecules, including the electromagnetic forces of attraction or repulsion which act between atoms and other types of neighbouring particles, e.g. atoms or ions. Intermolecular forces are weak relative to intramolecular forces – the forces which hold a molecule together. For example, the covalent bond, involving sharing electron pairs between atoms, is much stronger than the forces present between neighboring molecules. Both sets of forces are essential parts of force fields frequently used in molecular mechanics.

Solvation describes the interaction of a solvent with dissolved molecules. Both ionized and uncharged molecules interact strongly with a solvent, and the strength and nature of this interaction influence many properties of the solute, including solubility, reactivity, and color, as well as influencing the properties of the solvent such as its viscosity and density. If the attractive forces between the solvent and solute particles are greater than the attractive forces holding the solute particles together, the solvent particles pull the solute particles apart and surround them. The surrounded solute particles then move away from the solid solute and out into the solution. Ions are surrounded by a concentric shell of solvent. Solvation is the process of reorganizing solvent and solute molecules into solvation complexes and involves bond formation, hydrogen bonding, and van der Waals forces. Solvation of a solute by water is called hydration.

In molecular physics and chemistry, the van der Waals force is a distance-dependent interaction between atoms or molecules. Unlike ionic or covalent bonds, these attractions do not result from a chemical electronic bond; they are comparatively weak and therefore more susceptible to disturbance. The van der Waals force quickly vanishes at longer distances between interacting molecules.

London dispersion forces are a type of intermolecular force acting between atoms and molecules that are normally electrically symmetric; that is, the electrons are symmetrically distributed with respect to the nucleus. They are part of the van der Waals forces. The LDF is named after the German physicist Fritz London. They are the weakest intermolecular force.

Deposition is the geological process in which sediments, soil and rocks are added to a landform or landmass. Wind, ice, water, and gravity transport previously weathered surface material, which, at the loss of enough kinetic energy in the fluid, is deposited, building up layers of sediment.
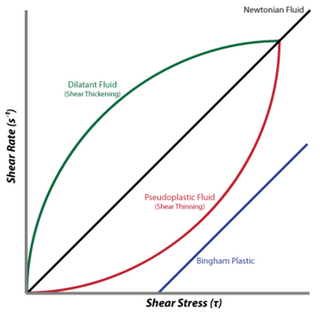
A dilatant material is one in which viscosity increases with the rate of shear strain. Such a shear thickening fluid, also known by the initialism STF, is an example of a non-Newtonian fluid. This behaviour is usually not observed in pure materials, but can occur in suspensions.

Adhesion is the tendency of dissimilar particles or surfaces to cling to one another.

Polyelectrolytes are polymers whose repeating units bear an electrolyte group. Polycations and polyanions are polyelectrolytes. These groups dissociate in aqueous solutions (water), making the polymers charged. Polyelectrolyte properties are thus similar to both electrolytes (salts) and polymers and are sometimes called polysalts. Like salts, their solutions are electrically conductive. Like polymers, their solutions are often viscous. Charged molecular chains, commonly present in soft matter systems, play a fundamental role in determining structure, stability and the interactions of various molecular assemblies. Theoretical approaches to describe their statistical properties differ profoundly from those of their electrically neutral counterparts, while technological and industrial fields exploit their unique properties. Many biological molecules are polyelectrolytes. For instance, polypeptides, glycosaminoglycans, and DNA are polyelectrolytes. Both natural and synthetic polyelectrolytes are used in a variety of industries.

In colloidal chemistry, flocculation is a process by which colloidal particles come out of suspension to sediment in the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from precipitation in that, prior to flocculation, colloids are merely suspended, under the form of a stable dispersion and are not truly dissolved in solution.
Quick clay, also known as Leda clay and Champlain Sea clay in Canada, is any of several distinctively sensitive glaciomarine clays found in Canada, Norway, Russia, Sweden, Finland, the United States, and other locations around the world. The clay is so unstable that when a mass of quick clay is subjected to sufficient stress, the material behavior may drastically change from that of a particulate material to that of a watery fluid. Landslides occur because of the sudden soil liquefaction caused by external solicitations such as vibrations induced by an earthquake, or massive rainfalls.

The nuclear force is a force that acts between hadrons, most commonly observed between protons and neutrons of atoms. Neutrons and protons, both nucleons, are affected by the nuclear force almost identically. Since protons have charge +1 e, they experience an electric force that tends to push them apart, but at short range the attractive nuclear force is strong enough to overcome the electrostatic force. The nuclear force binds nucleons into atomic nuclei.
In chemistry, a non-covalent interaction differs from a covalent bond in that it does not involve the sharing of electrons, but rather involves more dispersed variations of electromagnetic interactions between molecules or within a molecule. The chemical energy released in the formation of non-covalent interactions is typically on the order of 1–5 kcal/mol. Non-covalent interactions can be classified into different categories, such as electrostatic, π-effects, van der Waals forces, and hydrophobic effects.

In chemistry, pi stacking refers to the presumptive attractive, noncovalent pi interactions between the pi bonds of aromatic rings. According to some authors direct stacking of aromatic rings is electrostatically repulsive.

Particle agglomeration refers to the formation of assemblages in a suspension and represents a mechanism leading to the functional destabilization of colloidal systems. During this process, particles dispersed in the liquid phase stick to each other, and spontaneously form irregular particle assemblages, flocs, or agglomerates. This phenomenon is also referred to as coagulation or flocculation and such a suspension is also called unstable. Particle agglomeration can be induced by adding salts or other chemicals referred to as coagulant or flocculant.
An intramolecular force is any force that binds together the atoms making up a molecule or compound, not to be confused with intermolecular forces, which are the forces present between molecules. The subtle difference in the name comes from the Latin roots of English with inter meaning between or among and intra meaning inside. Chemical bonds are considered to be intramolecular forces which are often stronger than intermolecular forces present between non-bonding atoms or molecules.
Protein precipitation is widely used in downstream processing of biological products in order to concentrate proteins and purify them from various contaminants. For example, in the biotechnology industry protein precipitation is used to eliminate contaminants commonly contained in blood. The underlying mechanism of precipitation is to alter the solvation potential of the solvent, more specifically, by lowering the solubility of the solute by addition of a reagent.

Dispersive adhesion, also called adsorptive adhesion, is a mechanism for adhesion which attributes attractive forces between two materials to intermolecular interactions between molecules of each material. This mechanism is widely viewed as the most important of the five mechanisms of adhesion due to its presence in every type of adhesive system and its relative strength.
Adsorption of polyelectrolytes on solid substrates is a surface phenomenon where long-chained polymer molecules with charged groups bind to a surface that is charged in the opposite polarity. On the molecular level, the polymers do not actually bond to the surface, but tend to "stick" to the surface via intermolecular forces and the charges created by the dissociation of various side groups of the polymer. Because the polymer molecules are so long, they have a large amount of surface area with which to contact the surface and thus do not desorb as small molecules are likely to do. This means that adsorbed layers of polyelectrolytes form a very durable coating. Due to this important characteristic of polyelectrolyte layers they are used extensively in industry as flocculants, for solubilization, as supersorbers, antistatic agents, as oil recovery aids, as gelling aids in nutrition, additives in concrete, or for blood compatibility enhancement to name a few.
A depletion force is an effective attractive force that arises between large colloidal particles that are suspended in a dilute solution of depletants, which are smaller solutes that are preferentially excluded from the vicinity of the large particles. One of the earliest reports of depletion forces that lead to particle coagulation is that of Bondy, who observed the separation or "creaming" of rubber latex upon addition of polymer depletant molecules to solution. More generally, depletants can include polymers, micelles, osmolytes, ink, mud, or paint dispersed in a continuous phase.













