
Biomedical engineering (BME) or medical engineering is the application of engineering principles and design concepts to medicine and biology for healthcare purposes. BME is also traditionally logical sciences to advance health care treatment, including diagnosis, monitoring, and therapy. Also included under the scope of a biomedical engineer is the management of current medical equipment in hospitals while adhering to relevant industry standards. This involves procurement, routine testing, preventive maintenance, and making equipment recommendations, a role also known as a Biomedical Equipment Technician (BMET) or as clinical engineering.

A paramedic is a healthcare professional trained in the medical model, whose main role has historically been to respond to emergency calls for medical help outside of a hospital. Paramedics work as part of the emergency medical services (EMS), most often in ambulances. They also have roles in emergency medicine, primary care, transfer medicine and remote/offshore medicine. The scope of practice of a paramedic varies between countries, but generally includes autonomous decision making around the emergency care of patients.
Medical physics deals with the application of the concepts and methods of physics to the prevention, diagnosis and treatment of human diseases with a specific goal of improving human health and well-being. Since 2008, medical physics has been included as a health profession according to International Standard Classification of Occupation of the International Labour Organization.

Health informatics is the study and implementation of computer structures and algorithms to improve communication, understanding, and management of medical information. It can be view as branch of engineering and applied science.
A dietitian, medical dietitian, or dietician is an expert in identifying and treating disease-related malnutrition and in conducting medical nutrition therapy, for example designing an enteral tube feeding regimen or mitigating the effects of cancer cachexia. Many dietitians work in hospitals and usually see specific patients where a nutritional assessment and intervention has been requested by a doctor or nurse, for example if a patient has lost their ability to swallow or requires artificial nutrition due to intestinal failure. Dietitians are regulated healthcare professionals licensed to assess, diagnose, and treat such problems. In the United Kingdom, dietitian is a 'protected title', meaning identifying yourself as a dietitian without appropriate education and registration is prohibited by law.
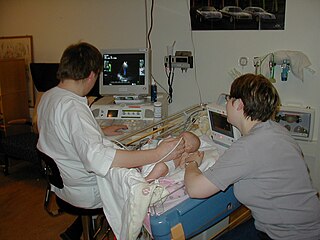
A sonographer is an allied healthcare professional who specializes in the use of ultrasonic imaging devices to produce diagnostic images, scans, videos or three-dimensional volumes of anatomy and diagnostic data. The requirements for clinical practice vary greatly by country. Sonography requires specialized education and skills to acquire, analyze and optimize information in the image. Due to the high levels of decisional latitude and diagnostic input, sonographers have a high degree of responsibility in the diagnostic process. Many countries require medical sonographers to have professional certification. Sonographers have core knowledge in ultrasound physics, cross-sectional anatomy, physiology, and pathology.

A medical laboratory scientist (MLS) or clinical laboratory scientist (CLS) or medical technologist (MT) performs diagnostic testing of blood and body fluids in clinical laboratories. The scope of a medical laboratory scientist's work begins with the receipt of patient or client specimens and terminates with the delivery of test results to physicians and other healthcare providers. The utility of clinical diagnostic testing relies squarely on the validity of test methodology. To this end, much of the work done by medical laboratory scientists involves ensuring specimen quality, interpreting test results, data-logging, testing control products, performing calibration, maintenance, validation, and troubleshooting of instrumentation as well as performing statistical analyses to verify the accuracy and repeatability of testing. Medical laboratory scientists may also assist healthcare providers with test selection and specimen collection and are responsible for prompt verbal delivery of critical lab results. Medical Laboratory Scientists in healthcare settings also play an important role in clinical diagnosis. An estimated 70% of medical decisions are based on laboratory test results and MLS contributions affect 95% of a health system's costs.
A biomedical engineering/equipment technician/technologist or biomedical engineering/equipment specialist is typically an electro-mechanical technician or technologist who ensures that medical equipment is well-maintained, properly configured, and safely functional. In healthcare environments, BMETs often work with or officiate as a biomedical and/or clinical engineer, since the career field has no legal distinction between engineers and engineering technicians/technologists.
In the United Kingdom, operating department practitioners (ODPs) are allied healthcare professionals who are involved in the planning and delivery of perioperative care. As the name suggests, they are primarily employed in surgical operating departments, but they may also work directly within a variety of acute clinical settings, including pre-hospital emergency care, emergency departments, intensive care units (ICUs), endoscopy suites, interventional radiology, cardiac catheter suites, obstetric theatres and reproductive medicine.
Health information management (HIM) is information management applied to health and health care. It is the practice of analyzing and protecting digital and traditional medical information vital to providing quality patient care. With the widespread computerization of health records, traditional (paper-based) records are being replaced with electronic health records (EHRs). The tools of health informatics and health information technology are continually improving to bring greater efficiency to information management in the health care sector.

Emergency medical personnel in the United Kingdom are people engaged in the provision of emergency medical services. This includes paramedics, emergency medical technicians and emergency care assistants. 'Paramedic' is a protected title, strictly regulated by the Health and Care Professions Council, although there is tendency for the public to use this term when referring to any member of ambulance staff.
The Association for the Advancement of Medical Instrumentation (AAMI) is an organization for advancing the development, and safe and effective use of medical technology founded in 1965 by Robert D. Hall Jr. and Robert J. Allen, President and Vice President respectively of Tech/Reps, Inc.. AAMI was created by the Tech/Reps' team as both a vehicle to help their clients introduce innovative medical devices into common medical practice and to set safety standards in both their design and usage. Dr. John Merrill of Peter Bent Brigham Hospital, and John Abele, Sales Manager of Advanced Instruments, Inc. joined with Hall and Allen to establish AAMI. Among the first members were Doctors Paul Dudley White, Michael Debakey, Adrian Kantrowitz, and the US Surgeon General. AAMI members now include decision makers in the medical technology profession—clinical engineers, biomedical equipment technicians, manufacturers, sterile processing professionals, researchers, quality assurance and regulatory affairs experts, and other healthcare technology management professionals. In 1966, AAMI was introduced to the public at large through MEDAC 66 held in Boston at which Drs. Debakey and Kantrowitz introduced the world's first artificial hearts and debated the merits of each.
A clinical coder—also known as clinical coding officer, diagnostic coder, medical coder, or nosologist—is a health information professional whose main duties are to analyse clinical statements and assign standardized codes using a classification system. The health data produced are an integral part of health information management, and are used by local and national governments, private healthcare organizations and international agencies for various purposes, including medical and health services research, epidemiological studies, health resource allocation, case mix management, public health programming, medical billing, and public education.
Medical equipment management is a term for the professionals who manage operations, analyze and improve utilization and safety, and support servicing healthcare technology. These healthcare technology managers are, much like other healthcare professionals referred to by various specialty or organizational hierarchy names.
National Board of Diving and Hyperbaric Medical Technology (NBDHMT), formally known as the National Association of Diving Technicians, is a non-profit organization devoted to the education and certification of qualified personnel in the fields of diving and hyperbaric medicine.

Engineering World Health (EWH) is a non-profit organization that works with hospitals and clinics that serve resource-poor communities of the developing world. EWH's focus is on the repair and maintenance of medical equipment - rather than donation - and on building local capacity to manage and maintain the equipment without international aid.

Biomedical sciences are a set of sciences applying portions of natural science or formal science, or both, to develop knowledge, interventions, or technology that are of use in healthcare or public health. Such disciplines as medical microbiology, clinical virology, clinical epidemiology, genetic epidemiology, and biomedical engineering are medical sciences. In explaining physiological mechanisms operating in pathological processes, however, pathophysiology can be regarded as basic science.

The Canadian Medical and Biological Engineering Society (CMBES) is a technical society representing the biomedical engineering community in Canada. CMBES is supported by its membership which consists of biomedical engineers, biomedical engineering technologists and students. CMBES also hosts an annual conference and regular webinars. It produces a number of publications including the Clinical Engineering Standards of Practice and a Newsletter. The Society's aims are twofold: scientific and educational: directed toward the advancement of the theory and practice of medical device technology; and professional: directed toward the advancement of all individuals in Canada who are engaged in interdisciplinary work involving engineering, the life sciences and medicine.
In its succinct definition, Healthcare Engineering is "engineering involved in all aspects of healthcare”. The term “engineering” in this definition covers all engineering disciplines such as biomedical, chemical, civil, computer, electrical, environmental, hospital architecture, industrial, information, materials, mechanical, software, and systems engineering.
Julian M. Goldman is an American physician and Medical Director of Biomedical Engineering at Mass General Brigham. He is the creator of Plug and Play Interoperability Research Program set up to promote innovation in patient safety and clinical care improve patient safety and make healthcare more efficient. He has been part of both the Harvard Medical School and Massachusetts General Hospital.









