Related Research Articles

Cluster sampling is a sampling plan used when mutually homogeneous yet internally heterogeneous groupings are evident in a statistical population. It is often used in marketing research. In this sampling plan, the total population is divided into these groups and a simple random sample of the groups is selected. The elements in each cluster are then sampled. If all elements in each sampled cluster are sampled, then this is referred to as a "one-stage" cluster sampling plan. If a simple random subsample of elements is selected within each of these groups, this is referred to as a "two-stage" cluster sampling plan. A common motivation for cluster sampling is to reduce the total number of interviews and costs given the desired accuracy. For a fixed sample size, the expected random error is smaller when most of the variation in the population is present internally within the groups, and not between the groups.

A randomized controlled trial is a type of scientific experiment or intervention study that aims to reduce certain sources of bias when testing the effectiveness of new treatments; this is accomplished by randomly allocating subjects to two or more groups, treating them differently, and then comparing them with respect to a measured response. One group—the experimental group—receives the intervention being assessed, while the other—usually called the control group—receives an alternative treatment, such as a placebo or no intervention. The groups are monitored under conditions of the trial design to determine the effectiveness of the experimental intervention, and efficacy is assessed in comparison to the control. There may be more than one treatment group or more than one control group.

Cochrane is a British international charitable organisation formed to organise medical research findings to facilitate evidence-based choices about health interventions involving health professionals, patients and policy makers. It includes 53 review groups that are based at research institutions worldwide. Cochrane has approximately 30,000 volunteer experts from around the world.
In a blind or blinded experiment, information which may influence the participants of the experiment is withheld until after the experiment is complete. Good blinding can reduce or eliminate experimental biases that arise from a participants' expectations, observer's effect on the participants, observer bias, confirmation bias, and other sources. A blind can be imposed on any participant of an experiment, including subjects, researchers, technicians, data analysts, and evaluators. In some cases, while blinding would be useful, it is impossible or unethical. For example, it is not possible to blind a patient to their treatment in a physical therapy intervention. A good clinical protocol ensures that blinding is as effective as possible within ethical and practical constraints.
Sir Richard Peto is an English statistician and epidemiologist who is Professor of Medical Statistics and Epidemiology at the University of Oxford, England.
Public health surveillance is, according to the World Health Organization (WHO), "the continuous, systematic collection, analysis and interpretation of health-related data needed for the planning, implementation, and evaluation of public health practice." Public health surveillance may be used to track emerging health-related issues at an early stage and find active solutions in a timely manner. Surveillance systems are generally called upon to provide information regarding when and where health problems are occurring and who is affected.
A hierarchy of evidence is a heuristic used to rank the relative strength of results obtained from scientific research. There is broad agreement on the relative strength of large-scale, epidemiological studies. More than 80 different hierarchies have been proposed for assessing medical evidence. The design of the study and the endpoints measured affect the strength of the evidence. In clinical research, the best evidence for treatment efficacy is mainly from meta-analyses of randomized controlled trials (RCTs). Typically, systematic reviews of completed, high-quality randomized controlled trials – such as those published by the Cochrane Collaboration – rank as the highest quality of evidence above observational studies, while expert opinion and anecdotal experience are at the bottom level of evidence quality. Evidence hierarchies are often applied in evidence-based practices and are integral to evidence-based medicine (EBM).
In medicine an intention-to-treat (ITT) analysis of the results of an experiment is based on the initial treatment assignment and not on the treatment eventually received. ITT analysis is intended to avoid various misleading artifacts that can arise in intervention research such as non-random attrition of participants from the study or crossover. ITT is also simpler than other forms of study design and analysis, because it does not require observation of compliance status for units assigned to different treatments or incorporation of compliance into the analysis. Although ITT analysis is widely employed in published clinical trials, it can be incorrectly described and there are some issues with its application. Furthermore, there is no consensus on how to carry out an ITT analysis in the presence of missing outcome data.
Zelen's design is an experimental design for randomized clinical trials proposed by Harvard School of Public Health statistician Marvin Zelen (1927-2014). In this design, patients are randomized to either the treatment or control group before giving informed consent. Because the group to which a given patient is assigned is known, consent can be sought conditionally.
A nurse-led clinic is any outpatient clinic that is run or managed by registered nurses, usually nurse practitioners or Clinical Nurse Specialists in the UK. Nurse-led clinics have assumed distinct roles over the years, and examples exist within hospital outpatient departments, public health clinics and independent practice environments.

In science, randomized experiments are the experiments that allow the greatest reliability and validity of statistical estimates of treatment effects. Randomization-based inference is especially important in experimental design and in survey sampling.
In epidemiology, Mendelian randomization is a method of using measured variation in genes of known function to examine the causal effect of a modifiable exposure on disease in observational studies. The design was first proposed in 1986 and subsequently described by Gray and Wheatley as a method for obtaining unbiased estimates of the effects of a putative causal variable without conducting a traditional randomised trial. These authors also coined the term Mendelian randomization. The design has a powerful control for reverse causation and confounding, which often impede or mislead epidemiological studies.
CONSORT encompasses various initiatives developed by the CONSORT Group to alleviate the problems arising from inadequate reporting of randomized controlled trials. It is part of the larger EQUATOR Network initiative to enhance the transparency and accuracy of reporting in research.
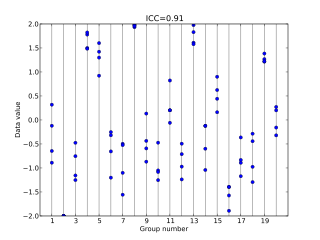
In statistics, the intraclass correlation, or the intraclass correlation coefficient (ICC), is a descriptive statistic that can be used when quantitative measurements are made on units that are organized into groups. It describes how strongly units in the same group resemble each other. While it is viewed as a type of correlation, unlike most other correlation measures it operates on data structured as groups, rather than data structured as paired observations.
Exercise prescription commonly refers to the specific plan of fitness-related activities that are designed for a specified purpose, which is often developed by a fitness or rehabilitation specialist for the client or patient. Due to the specific and unique needs and interests of the client/patient, the goal of exercise prescription should be focused on motivation and customization, thus making achieving goals more likely to become successful.
In statistics, the design effect is an adjustment used in some kinds of studies, such as ones that use cluster sampling or cluster randomised controlled trial, to allow for the design structure. The adjustment inflates the variance of parameter estimates, and therefore their standard errors, which is necessary to allow for correlations among clusters of observations. It is similar to the variance inflation factor and is used in sample size calculations. The term was introduced by Leslie Kish in 1965.
Three Degrees of Influence is a theory in the realm of social networks, proposed by Nicholas A. Christakis and James H. Fowler in 2007. It has since been explored by scientists in numerous disciplines using diverse statistical, psychological, sociological, and biological approaches.

PRISMA is an evidence-based minimum set of items aimed at helping authors to report a wide array of systematic reviews and meta-analyses that assess the benefits and harms of a health care intervention. PRISMA focuses on ways in which authors can ensure a transparent and complete reporting of this type of research. The PRISMA standard supersedes the QUOROM standard.
Associations have been shown between vitamin D levels and several respiratory tract infections suggesting that vitamin D deficiency may predispose to infection. Outbreaks of respiratory infections occur predominantly during months associated with lower exposure to the sun. The Institute of Medicine concluded in a 2011 report that the existing data were "not consistently supportive of a causal role" for vitamin D in reducing the risk of infection. Other studies suggest that vitamin D supplementation can provide a protective role in reducing the incidence or severity of respiratory infections.
A stepped-wedge trial is a type of randomised controlled trial, a scientific experiment which is structured to reduce bias when testing new medical treatments, social interventions, or other testable hypotheses. In a traditional RCT, a part of the participants in the experiment are simultaneously and randomly assigned to a group that receives the treatment and another part to a group that does not. In an SWT, typically a logistical constraint prevents the simultaneous treatment of some participants, and instead, all or most participants receive the treatment in waves or "steps".
References
- 1 2 Bland JM (2004). "Cluster randomised trials in the medical literature: two bibliometric surveys". BMC Med Res Methodol. 4: 21. doi:10.1186/1471-2288-4-21. PMC 515302 . PMID 15310402.
- 1 2 Campbell MK, Elbourne DR, Altman DG, CONSORT group (2004). "CONSORT statement: extension to cluster randomised trials". BMJ. 328 (7441): 702–8. doi:10.1136/bmj.328.7441.702. PMC 381234 . PMID 15031246.
- ↑ Murray DM, Varnell SP, Blitstein JL (2004). "Design and analysis of group-randomized trials: a review of recent methodological developments". Am J Public Health. 94 (3): 423–32. doi:10.2105/AJPH.94.3.423. PMC 1448268 . PMID 14998806.
- ↑ Patton GC, Bond L, Carlin JB, Thomas L, Butler H, Glover S, Catalano R, Bowes G (2006). "Promoting social inclusion in schools: a group-randomized trial of effects on student health risk behavior and well-being". Am J Public Health. 96 (9): 1582–7. doi:10.2105/AJPH.2004.047399. PMC 1551970 . PMID 16873760.
- ↑ Boruch R, May H, Turner H, Lavenberg J, Petrosino A, De Moya D, Grimshaw J, Foley E (2004). "Estimating the effects of interventions that are deployed in many places: place-randomized trials". American Behavioral Scientist . 47 (5): 608–633. doi:10.1177/0002764203259291.CS1 maint: discouraged parameter (link)[ permanent dead link ]
- 1 2 Murray, David M.; Taljaard, Monica; Turner, Elizabeth L.; George, Stephanie M. (2020). "Essential Ingredients and Innovations in the Design and Analysis of Group-Randomized Trials". Annual Review of Public Health. 41: 1–19. doi: 10.1146/annurev-publhealth-040119-094027 . PMID 31869281.
- ↑ Edwards SJ, Braunholtz DA, Lilford RJ, Stevens AJ (1999). "Ethical issues in the design and conduct of cluster randomised controlled trials". BMJ. 318 (7195): 1407–9. doi:10.1136/bmj.318.7195.1407. PMC 1115783 . PMID 10334756.
- ↑ Sampling People, Networks and Records coursers course (worth finding a better reference)