Urea, also called carbamide, is an organic compound with chemical formula CO(NH2)2. This amide has two amino groups joined by a carbonyl functional group. It is thus the simplest amide of carbamic acid.

A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment or hand-tool methods.

Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer.

Corrosion is a natural process that converts a refined metal into a more chemically stable oxide. It is the gradual deterioration of materials by chemical or electrochemical reaction with their environment. Corrosion engineering is the field dedicated to controlling and preventing corrosion.

In metallurgy, a flux is a chemical cleaning agent, flowing agent, or purifying agent. Fluxes may have more than one function at a time. They are used in both extractive metallurgy and metal joining.

Barium sulfate (or sulphate) is the inorganic compound with the chemical formula BaSO4. It is a white crystalline solid that is odorless and insoluble in water. It occurs as the mineral barite, which is the main commercial source of barium and materials prepared from it. Its opaque white appearance and its high density are exploited in its main applications.

Electrophoretic deposition (EPD), is a term for a broad range of industrial processes which includes electrocoating, cathodic electrodeposition, anodic electrodeposition, and electrophoretic coating, or electrophoretic painting. A characteristic feature of this process is that colloidal particles suspended in a liquid medium migrate under the influence of an electric field (electrophoresis) and are deposited onto an electrode. All colloidal particles that can be used to form stable suspensions and that can carry a charge can be used in electrophoretic deposition. This includes materials such as polymers, pigments, dyes, ceramics and metals.

Sulfamic acid, also known as amidosulfonic acid, amidosulfuric acid, aminosulfonic acid, sulphamic acid and sulfamidic acid, is a molecular compound with the formula H3NSO3. This colourless, water-soluble compound finds many applications. Sulfamic acid melts at 205 °C before decomposing at higher temperatures to water, sulfur trioxide, sulfur dioxide and nitrogen.

Organic fertilizers are fertilizers that are naturally produced. Fertilizers are materials that can be added to soil or plants, in order to provide nutrients and sustain growth. Typical organic fertilizers include all animal waste including meat processing waste, manure, slurry, and guano; plus plant based fertilizers such as compost; and biosolids. Inorganic "organic fertilizers" include minerals and ash. The organic-mess refers to the Principles of Organic Agriculture, which determines whether a fertilizer can be used for commercial organic agriculture, not whether the fertilizer consists of organic compounds.

Powder coating is a type of coating that is applied as a free-flowing, dry powder. Unlike conventional liquid paint, which is delivered via an evaporating solvent, powder coating is typically applied electrostatically and then cured under heat or with ultraviolet light. The powder may be a thermoplastic or a thermoset polymer. It is usually used to create a thick, tough finish that is more durable than conventional paint. Powder coating is mainly used for coating of metal objects, particularly those subject to rough use. Advancements in powder coating technology like UV-curable powder coatings allow for other materials such as plastics, composites, carbon fiber, and MDF to be powder coated, as little heat or oven dwell time is required to process them.
Hydrophobic soil is a soil whose particles repel water. The layer of hydrophobicity is commonly found at or a few centimeters below the surface, parallel to the soil profile. This layer can vary in thickness and abundance and is typically covered by a layer of ash or burned soil.
Metabolic wastes or excrements are substances left over from metabolic processes (such as cellular respiration) which cannot be used by the organism (they are surplus or toxic), and must therefore be excreted. This includes nitrogen compounds, water, CO2, phosphates, sulphates, etc. Animals treat these compounds as excretes. Plants have metabolic pathways which transforms some of them (primarily the oxygen compounds) into useful substances.
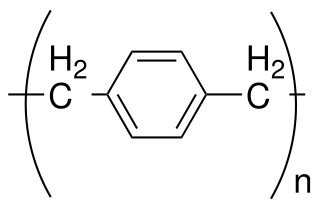
Parylene is the common name of a polymer whose backbone consists of para-benzenediyl rings −C
6H
4− connected by 1,2-ethanediyl bridges −CH
2−CH
2−. It can be obtained by polymerization of para-xylyleneH
2C=C
6H
4=CH
2.
Stone sealing is the application of a surface treatment to products constructed of natural stone to retard staining and corrosion. All bulk natural stone is riddled with interconnected capillary channels that permit penetration by liquids and gases. This is true for igneous rock types such as granite and basalt, metamorphic rocks such as marble and slate, and sedimentary rocks such as limestone, travertine, and sandstone. These porous channels act like a sponge, and capillary action draws in liquids over time, along with any dissolved salts and other solutes. Very porous stone, such as sandstone absorb liquids relatively quickly, while denser igneous stones such as granite are significantly less porous; they absorb smaller volumes, and more slowly, especially when absorbing viscous liquids.
A controlled-release fertiliser (CRF) is a granulated fertiliser that releases nutrients gradually into the soil. Controlled-release fertilizer is also known as controlled-availability fertilizer, delayed-release fertilizer, metered-release fertilizer, or slow-acting fertilizer. Usually CRF refers to nitrogen-based fertilizers. Slow- and controlled-release involve only 0.15% of the fertilizer market (1995).

Sable Chemical Industries Limited is the sole manufacturer of ammonium nitrate (NH4NO3) in Zimbabwe.
Urea (46-0-0) accounts for more than fifty percent of the world's nitrogenous fertilizers. It is found in granular or prill form, which allows urea to be easily stored, transported and applied in agricultural settings. It is also the cheapest form of granular nitrogen fertilizer. Since urea is not an oxidizer at standard temperature and pressure, it is safer to handle and less of a security risk than other common nitrogen fertilizers, such as ammonium nitrate. However, if urea is applied to the soil surface, a meaningful fraction of applied fertilizer nitrogen may be lost to the atmosphere as ammonia gas; this only occurs under certain conditions.
Adsorption of polyelectrolytes on solid substrates is a surface phenomenon where long-chained polymer molecules with charged groups bind to a surface that is charged in the opposite polarity. On the molecular level, the polymers do not actually bond to the surface, but tend to "stick" to the surface via intermolecular forces and the charges created by the dissociation of various side groups of the polymer. Because the polymer molecules are so long, they have a large amount of surface area with which to contact the surface and thus do not desorb as small molecules are likely to do. This means that adsorbed layers of polyelectrolytes form a very durable coating. Due to this important characteristic of polyelectrolyte layers they are used extensively in industry as flocculants, for solubilization, as supersorbers, antistatic agents, as oil recovery aids, as gelling aids in nutrition, additives in concrete, or for blood compatibility enhancement to name a few.
The surface chemistry of paper is responsible for many important paper properties, such as gloss, waterproofing, and printability. Many components are used in the paper-making process that affect the surface.

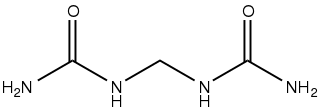
Glycoluril is an organic chemical composed of two cyclic urea groups joined across the same two-carbon chain. It is a white powder that has been used in water treatment, in paints and coatings, and occasionally as a slow-release fertilizer.












