
An explosive is a reactive substance that contains a great amount of potential energy that can produce an explosion if released suddenly, usually accompanied by the production of light, heat, sound, and pressure. An explosive charge is a measured quantity of explosive material, which may either be composed solely of one ingredient or be a mixture containing at least two substances.

Paraffin wax is a soft colorless solid derived from petroleum, coal, or oil shale that consists of a mixture of hydrocarbon molecules containing between 20 and 40 carbon atoms. It is solid at room temperature and begins to melt above approximately 37 °C (99 °F), and its boiling point is above 370 °C (698 °F). Common applications for paraffin wax include lubrication, electrical insulation, and candles; dyed paraffin wax can be made into crayons.
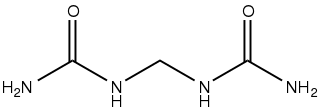
Urea, also called carbamide, is an organic compound with chemical formula CO(NH2)2. This amide has two amino groups joined by a carbonyl functional group. It is thus the simplest amide of carbamic acid.

A fertilizer or fertiliser is any material of natural or synthetic origin that is applied to soil or to plant tissues to supply plant nutrients. Fertilizers may be distinct from liming materials or other non-nutrient soil amendments. Many sources of fertilizer exist, both natural and industrially produced. For most modern agricultural practices, fertilization focuses on three main macro nutrients: nitrogen (N), phosphorus (P), and potassium (K) with occasional addition of supplements like rock flour for micronutrients. Farmers apply these fertilizers in a variety of ways: through dry or pelletized or liquid application processes, using large agricultural equipment, or hand-tool methods.

Ammonium nitrate is a chemical compound with the formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer.

ANFO ( AN-foh) (or AN/FO, for ammonium nitrate/fuel oil) is a widely used bulk industrial high explosive. It consists of 94% porous prilled ammonium nitrate (NH4NO3) (AN), which acts as the oxidizing agent and absorbent for the fuel, and 6% number 2 fuel oil (FO). The use of ANFO originated in the 1950s.
UAN is a solution of urea and ammonium nitrate in water used as a fertilizer. The combination of urea and ammonium nitrate has an extremely low critical relative humidity and can therefore only be used in liquid fertilizers. The most commonly used grade of these fertilizer solutions is UAN 32.0.0 (32%N) known as UN32 or UN-32, which consists of 45% ammonium nitrate, 35% urea and only 20% water. Other grades are UAN 28, UAN 30 and UAN 18. The solutions are quite corrosive towards mild steel and are therefore generally equipped with a corrosion inhibitor to protect tanks, pipelines, nozzles, etc. Urea–ammonium nitrate solutions should not be combined with calcium ammonium nitrate (CAN-17) or other solutions prepared from calcium nitrate. A thick, milky-white insoluble precipitate forms that may plug nozzles.

Calcium nitrate are inorganic compounds with the formula Ca(NO3)2(H2O)x. The anhydrous compound, which is rarely encountered, absorbs moisture from the air to give the tetrahydrate. Both anhydrous and hydrated forms are colourless salts. Hydrated calcium nitrate, also called Norgessalpeter (Norwegian salpeter), is mainly used as a component in fertilizers, but it has other applications. Nitrocalcite is the name for a mineral which is a hydrated calcium nitrate that forms as an efflorescence where manure contacts concrete or limestone in a dry environment as in stables or caverns. A variety of related salts are known including calcium ammonium nitrate decahydrate and calcium potassium nitrate decahydrate.

Sulfamic acid, also known as amidosulfonic acid, amidosulfuric acid, aminosulfonic acid, sulphamic acid and sulfamidic acid, is a molecular compound with the formula H3NSO3. This colourless, water-soluble compound finds many applications. Sulfamic acid melts at 205 °C before decomposing at higher temperatures to water, sulfur trioxide, sulfur dioxide and nitrogen.

National Fertilizers Limited (NFL) is an Indian central public sector undertaking and the largest government-owned-Urea fertilizer-producer in India. It is a Navratna company, with the Government of India owning a majority stake.
The critical relative humidity (CRH) of a salt is defined as the relative humidity of the surrounding atmosphere at which the material begins to absorb moisture from the atmosphere and below which it will not absorb atmospheric moisture.

Urea nitrate is a fertilizer-based high explosive that has been used in improvised explosive devices in Afghanistan, Pakistan, Iraq, and various terrorist acts elsewhere in the world such as in the 1993 World Trade Center bombings. It has a destructive power similar to better-known ammonium nitrate explosives, with a velocity of detonation between 3,400 m/s (11,155 ft/s) and 4,700 m/s (15,420 ft/s). It has chemical formula of CH5N3O4 or (NH2)2COHNO3.
A controlled-release fertiliser (CRF) is a granulated fertiliser that releases nutrients gradually into the soil. Controlled-release fertilizer is also known as controlled-availability fertilizer, delayed-release fertilizer, metered-release fertilizer, or slow-acting fertilizer. Usually CRF refers to nitrogen-based fertilizers. Slow- and controlled-release involve only 0.15% of the fertilizer market (1995).

Sable Chemical Industries Limited is the sole manufacturer of ammonium nitrate (NH4NO3) in Zimbabwe.
Urea (46-0-0) accounts for more than fifty percent of the world's nitrogenous fertilizers. It is found in granular or prill form, which allows urea to be easily stored, transported and applied in agricultural settings. It is also the cheapest form of granular nitrogen fertilizer. Since urea is not an oxidizer at standard temperature and pressure, it is safer to handle and less of a security risk than other common nitrogen fertilizers, such as ammonium nitrate. However, if urea is applied to the soil surface, a meaningful fraction of applied fertilizer nitrogen may be lost to the atmosphere as ammonia gas; this only occurs under certain conditions.
Explosive materials are produced in numerous physical forms for their use in mining, engineering, or military applications. The different physical forms and fabrication methods are grouped together in several use forms of explosives.
Coated urea fertilizers are a group of controlled release fertilizers consisting of prills of urea coated in less-soluble chemicals such as sulfur, polymers, other products or a combination. These fertilizers mitigate some of the negative aspects of urea fertilization, such as fertilizer burn. The coatings release the urea either when penetrated by water, as with sulfur, or when broken down, as with polymers.

Azot, also known as Cherkaskyi Azot after Cherkasy, the location of its chemical plant, is one of the biggest manufacturers of nitrogen fertilizers in Ukraine. Ukrainian oligarch Dmytro Firtash's chemical-industry holding corporation, Ostchem Holding, manages the company as well as several other fertilizer manufacturers in Ukraine and other post-Soviet states.
Fertilizer Corporation of India Limited (FCIL) is a public sector undertaking in India under the ownership of Ministry of Chemicals and Fertilizers, Government of India.

The Bosch–Meiser process is an industrial process, which was patented in 1922 and named after its discoverers, the German chemists Carl Bosch and Wilhelm Meiser for the large-scale manufacturing of urea, a valuable nitrogenous chemical.












