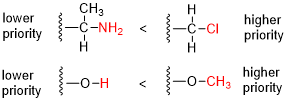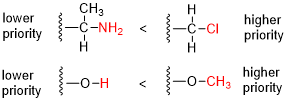
In organic chemistry, the Cahn–Ingold–Prelog (CIP) sequence rules are a standard process to completely and unequivocally name a stereoisomer of a molecule. The purpose of the CIP system is to assign an R or S descriptor to each stereocenter and an E or Z descriptor to each double bond so that the configuration of the entire molecule can be specified uniquely by including the descriptors in its systematic name. A molecule may contain any number of stereocenters and any number of double bonds, and each usually gives rise to two possible isomers. A molecule with an integer n describing the number of stereocenters will usually have 2n stereoisomers, and 2n−1 diastereomers each having an associated pair of enantiomers. The CIP sequence rules contribute to the precise naming of every stereoisomer of every organic molecule with all atoms of ligancy of fewer than 4.

The International Union of Pure and Applied Chemistry is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. This administrative office is headed by IUPAC's executive director, currently Greta Heydenrych.

In chemistry, an enantiomer – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are non-superposable onto their own mirror image. Enantiomers are much like one's right and left hands; without mirroring one of them, hands cannot be superposed onto each other. No amount of reorientation in three spatial dimensions will allow the four unique groups on the chiral carbon to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has. Stereoisomers include both enantiomers and diastereomers.
In chemistry, the standard state of a material is a reference point used to calculate its properties under different conditions. A superscript circle ° or a Plimsoll (⦵) character is used to designate a thermodynamic quantity in the standard state, such as change in enthalpy (ΔH°), change in entropy (ΔS°), or change in Gibbs free energy (ΔG°). The degree symbol has become widespread, although the Plimsoll is recommended in standards, see discussion about typesetting below.
Nomenclature is a system of names or terms, or the rules for forming these terms in a particular field of arts or sciences. The principles of naming vary from the relatively informal conventions of everyday speech to the internationally agreed principles, rules and recommendations that govern the formation and use of the specialist terminology used in scientific and any other disciplines.
ISO 31 is a superseded international standard concerning physical quantities, units of measurement, their interrelationships and their presentation. It was revised and replaced by ISO/IEC 80000.
In chemistry an activated complex is defined by the International Union of Pure and Applied Chemistry (IUPAC) as "that assembly of atoms which corresponds to an arbitrary infinitesimally small region at or near the col of a potential energy surface". In other words, it refers to a collection of intermediate structures in a chemical reaction when bonds are breaking and new bonds are forming. It therefore represents not one defined state, but rather a range of transient configurations that a collection of atoms passes through in between clearly defined products and reactants.
The coefficient of haze is a measurement of visibility interference in the atmosphere.
A chemical nomenclature is a set of rules to generate systematic names for chemical compounds. The nomenclature used most frequently worldwide is the one created and developed by the International Union of Pure and Applied Chemistry (IUPAC).
In chemical nomenclature, the IUPAC nomenclature of inorganic chemistry is a systematic method of naming inorganic chemical compounds, as recommended by the International Union of Pure and Applied Chemistry (IUPAC). It is published in Nomenclature of Inorganic Chemistry. Ideally, every inorganic compound should have a name from which an unambiguous formula can be determined. There is also an IUPAC nomenclature of organic chemistry.

Quantities, Units and Symbols in Physical Chemistry, also known as the Green Book, is a compilation of terms and symbols widely used in the field of physical chemistry. It also includes a table of physical constants, tables listing the properties of elementary particles, chemical elements, and nuclides, and information about conversion factors that are commonly used in physical chemistry. The Green Book is published by the International Union of Pure and Applied Chemistry (IUPAC) and is based on published, citeable sources. Information in the Green Book is synthesized from recommendations made by IUPAC, the International Union of Pure and Applied Physics (IUPAP) and the International Organization for Standardization (ISO), including recommendations listed in the IUPAP Red Book Symbols, Units, Nomenclature and Fundamental Constants in Physics and in the ISO 31 standards.
In chemistry, the equivalent concentration or normality of a solution is defined as the molar concentration ci divided by an equivalence factor or n-factor feq:

Nomenclature of Organic Chemistry, commonly referred to by chemists as the Blue Book, is a collection of recommendations on organic chemical nomenclature published at irregular intervals by the International Union of Pure and Applied Chemistry (IUPAC). A full edition was published in 1979, an abridged and updated version of which was published in 1993 as A Guide to IUPAC Nomenclature of Organic Compounds. Both of these are now out-of-print in their paper versions, but are available free of charge in electronic versions. After the release of a draft version for public comment in 2004 and the publication of several revised sections in the journal Pure and Applied Chemistry, a fully revised edition was published in print in 2013 and its online version is also available.
In organic chemistry, Hantzsch–Widman nomenclature, also called the extended Hantzsch–Widman system, is a type of systematic chemical nomenclature used for naming heterocyclic parent hydrides having no more than ten ring members. Some common heterocyclic compounds have retained names that do not follow the Hantzsch–Widman pattern.
An equivalent is the amount of a substance that reacts with an arbitrary amount of another substance in a given chemical reaction. It is an archaic unit of measurement that was used in chemistry and the biological sciences. The mass of an equivalent is called its equivalent weight.
The International Union of Pure and Applied Chemistry (IUPAC) publishes many books which contain its complete list of definitions. The definitions are divided initially into seven IUPC Colour Books: Gold, Green, Blue, Purple, Orange, White, and Red. There is also an eighth book, the "Silver Book".
The Compendium of Macromolecular Nomenclature, by the International Union of Pure and Applied Chemistry (IUPAC), provides definition of polymer related terms and rules of nomenclature of polymers. It is referred to as the Purple Book. It was published in 1991 (ISBN 0-63202-8475) by Blackwell Science. The author of this book is W.V. Metanomski.
In chemistry, the molar absorption coefficient or molar attenuation coefficient is a measurement of how strongly a chemical species absorbs, and thereby attenuates, light at a given wavelength. It is an intrinsic property of the species. The SI unit of molar absorption coefficient is the square metre per mole, but in practice, quantities are usually expressed in terms of M−1⋅cm−1 or L⋅mol−1⋅cm−1. In older literature, the cm2/mol is sometimes used; 1 M−1⋅cm−1 equals 1000 cm2/mol. The molar absorption coefficient is also known as the molar extinction coefficient and molar absorptivity, but the use of these alternative terms has been discouraged by the IUPAC.

The International Union of Pure and Applied Chemistry (IUPAC) has published four sets of rules to standardize chemical nomenclature.

In chromatography, resolution is a measure of the separation of two peaks of different retention time t in a chromatogram.