Darbepoetin alfa (INN) is a re-engineered form of erythropoietin containing 5 amino acid changes resulting in the creation of 2 new sites for N-linked carbohydrate addition. It has a 3-fold longer serum half-life compared to epoetin alpha and epoetin beta. It stimulates erythropoiesis by the same mechanism as rHuEpo and is used to treat anemia, commonly associated with chronic kidney failure and cancer chemotherapy. Darbepoetin is marketed by Amgen under the trade name Aranesp.

Erythropoietin, also known as erythropoetin, haematopoietin, or haemopoietin, is a glycoprotein cytokine secreted mainly by the kidneys in response to cellular hypoxia; it stimulates red blood cell production (erythropoiesis) in the bone marrow. Low levels of EPO are constantly secreted in sufficient quantities to compensate for normal red blood cell turnover. Common causes of cellular hypoxia resulting in elevated levels of EPO include any anemia, and hypoxemia due to chronic lung disease.
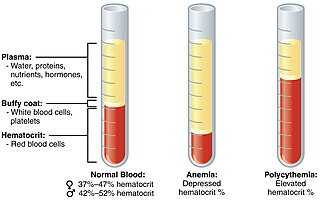
Polycythemia is a laboratory finding in which the hematocrit and/or hemoglobin concentration are increased in the blood. Polycythemia is sometimes called erythrocytosis, and there is significant overlap in the two findings, but the terms are not the same: polycythemia describes any increase in hematocrit and/or hemoglobin, while erythrocytosis describes an increase specifically in the number of red blood cells in the blood.
Blood doping is a form of doping in which the number of red blood cells in the bloodstream is boosted in order to enhance athletic performance. Because such blood cells carry oxygen from the lungs to the muscles, a higher concentration in the blood can improve an athlete's aerobic capacity (VO2 max) and endurance. Blood doping can be achieved by making the body produce more red blood cells itself using drugs, giving blood transfusions either from another person or back to the same individual, or by using blood substitutes.
Athanasia Tsoumeleka is a Greek race walker, who won a gold medal at the 2004 Summer Olympics in Athens.
Epoetin alfa is a human erythropoietin produced in cell culture using recombinant DNA technology. Authorised by the European Medicines Agency on 28 August 2007, it stimulates erythropoiesis and is used to treat anemia, commonly associated with chronic kidney failure and cancer chemotherapy.

Riccardo Riccò is an Italian professional road bicycle racer, who is suspended from all competition until 2024. He was previously ejected from the 2008 Tour de France for doping violations and suspended. Riccò returned to competition in late 2010, but in February 2011 he was fired by his team, Vacansoleil–DCM, after he became seriously ill allegedly through a self-administered autologous blood transfusion. He then signed to UCI Continental team Meridiana–Kamen.
Erythropoiesis-stimulating agents (ESA) are medications which stimulate the bone marrow to make red blood cells. They are used to treat anemia due to end stage kidney disease, chemotherapy, major surgery, or certain treatments in HIV/AIDS. In these situations they decrease the need for blood transfusions. The different agents are more or less equivalent. They are given by injection.
Epoetin beta (INN), sold under the brand name Neorecormon among others, is a synthetic, recombinant form of erythropoietin, a protein that promotes the production of red blood cells. It is an erythropoiesis-stimulating agent (ESA) that is used to treat anemia, commonly associated with chronic kidney failure and cancer chemotherapy.
Methoxy polyethylene glycol-epoetin beta, sold under the brand name Mircera, is a long-acting erythropoietin receptor activator (CERA) used for the treatment of anaemia associated with chronic kidney disease. It is the first approved, chemically modified erythropoiesis-stimulating agent (ESA).

Robert Provenzano is an American nephrologist. He is also an Associate Clinical Professor of Medicine at Wayne State University School of Medicine.
Matteo Priamo is an Italian professional road bicycle racer, previously of UCI Professional Continental team CSF Group–Navigare.
Don H. Catlin is an anti-doping scientist and one of the founders of modern drug-testing in sport.
Eugene Goldwasser was an American biochemist at the University of Chicago who identified erythropoietin, a hormone that plays a vital role in the synthesis of red blood cells. After sharing the minute quantities that he had been able to isolate with researchers at the biotechnology firm Amgen, that company was able to use genetic engineering technology to produce useful amounts of EPO as a drug to treat anemia that has achieved substantial financial success, but that has also been used by athletes as a performance-enhancing drug.
Peginesatide, developed by Affymax and Takeda, is an erythropoietic agent, a functional analog of erythropoietin.
CSL Vifor is a global specialty pharmaceuticals company in the treatment areas of iron deficiency, dialysis, nephrology & rare disease. It is headquartered in Switzerland and consists of CSL Vifor, Vifor Fresenius Medical Care Renal Pharma (VFMCRP) and Sanifit Therapeutics.

Roxadustat, sold under the brand name Evrenzo, is an anti-anemia medication. Roxadustat is a HIF prolyl-hydroxylase inhibitor that increases endogenous production of erythropoietin and stimulates production of hemoglobin and red blood cells. It was investigated in clinical trials for the treatment of anemia caused by chronic kidney disease (CKD). It is taken by mouth. The drug was developed by FibroGen, in partnership with AstraZeneca.
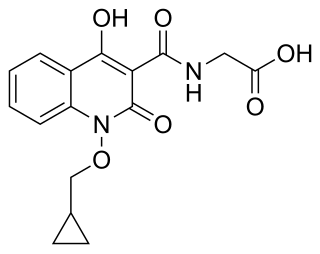
Desidustat is a drug for the treatment of anemia of chronic kidney disease. This drug with the brand name Oxemia is discovered and developed by Zydus Life Sciences. Desidustat reduces the requirement of recombinant erythropoietin requirement in anemia, and decreases EPO-resistance, by reducing IL-6, IL-1β, and anti-EPO antibodies. The subject expert committee of CDSCO has recommended the grant of permission for manufacturing and marketing of Desidustat 25 mg and 50 mg tablets in India,based on some conditions related to package insert, phase 4 protocols, prescription details, and GCP. Clinical trials on desidustat have been done in India and Australia. In a Phase 2, randomized, double-blind, 6-week, placebo-controlled, dose-ranging, safety and efficacy study, a mean hemoglobin increase of 1.57, 2.22, and 2.92 g/dL in desidustat 100, 150, and 200 mg arms, respectively, was observed. The Phase 3 clinical trials were conducted in chronic kidney disease patients which were not on dialysis as well as on dialysis. Desidustat is developed for the treatment of anemia as an oral tablet, where currently injections of erythropoietin and its analogues are drugs of choice. Desidustat is a HIF prolyl-hydroxylase inhibitor. In preclinical studies, effects of desidustat was assessed in normal and nephrectomized rats, and in chemotherapy-induced anemia. Desidustat demonstrated hematinic potential by combined effects on endogenous erythropoietin release and efficient iron utilization. Desidustat can also be useful in treatment of anemia of inflammation since it causes efficient erythropoiesis and hepcidin downregulation. In January 2020, Zydus entered into licensing agreement with China Medical System (CMS) Holdings for development and commercialization of desidustat in Greater China. Under the license agreement, CMS will pay Zydus an initial upfront payment, regulatory milestones, sales milestones and royalties on net sales of the product. CMS will be responsible for development, registration and commercialization of desidustat in Greater China. It has been observed that desidustat protects against acute and chronic kidney injury by reducing inflammatory cytokines like IL-6 and oxidative stress A clinical trial to evaluate the efficacy and safety of desidustat tablet for the management of Covid-19 patients is ongoing in Mexico, wherein desidustat has shown to prevent acute respiratory distress syndrome (ARDS) by inhibiting IL-6. Zydus has also received approval from the US FDA to initiate clinical trials of desidustat in chemotherapy Induced anemia (CIA).






