
A calorimeter is an object used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. Differential scanning calorimeters, isothermal micro calorimeters, titration calorimeters and accelerated rate calorimeters are among the most common types. A simple calorimeter just consists of a thermometer attached to a metal container full of water suspended above a combustion chamber. It is one of the measurement devices used in the study of thermodynamics, chemistry, and biochemistry.
Instrumentation is a collective term for measuring instruments, used for indicating, measuring and recording physical quantities. It is also a field of study about the art and science about making measurement instruments, involving the related areas of metrology, automation, and control theory. The term has its origins in the art and science of scientific instrument-making.

Differential scanning calorimetry (DSC) is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature. Both the sample and reference are maintained at nearly the same temperature throughout the experiment. Generally, the temperature program for a DSC analysis is designed such that the sample holder temperature increases linearly as a function of time. The reference sample should have a well-defined heat capacity over the range of temperatures to be scanned. Additionally, the reference sample must be stable, of high purity, and must not experience much change across the temperature scan. Typically, reference standards have been metals such as indium, tin, bismuth, and lead, but other standards such as polyethylene and fatty acids have been proposed to study polymers and organic compounds, respectively.

A pressurized water reactor (PWR) is a type of light-water nuclear reactor. PWRs constitute the large majority of the world's nuclear power plants. In a PWR, the primary coolant (water) is pumped under high pressure to the reactor core where it is heated by the energy released by the fission of atoms. The heated, high pressure water then flows to a steam generator, where it transfers its thermal energy to lower pressure water of a secondary system where steam is generated. The steam then drives turbines, which spin an electric generator. In contrast to a boiling water reactor (BWR), pressure in the primary coolant loop prevents the water from boiling within the reactor. All light-water reactors use ordinary water as both coolant and neutron moderator. Most use anywhere from two to four vertically mounted steam generators; VVER reactors use horizontal steam generators.
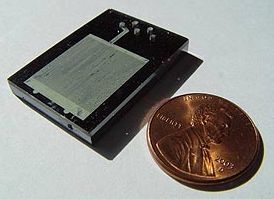
A microreactor or microstructured reactor or microchannel reactor is a device in which chemical reactions take place in a confinement with typical lateral dimensions below 1 mm; the most typical form of such confinement are microchannels. Microreactors are studied in the field of micro process engineering, together with other devices in which physical processes occur. The microreactor is usually a continuous flow reactor. Microreactors offer many advantages over conventional scale reactors, including vast improvements in energy efficiency, reaction speed and yield, safety, reliability, scalability, on-site/on-demand production, and a much finer degree of process control.

Laboratory robotics is the act of using robots in biology, chemistry or engineering labs. For example, pharmaceutical companies employ robots to move biological or chemical samples around to synthesize novel chemical entities or to test pharmaceutical value of existing chemical matter. Advanced laboratory robotics can be used to completely automate the process of science, as in the Robot Scientist project.

A chemical reactor is an enclosed volume in which a chemical reaction takes place. In chemical engineering, it is generally understood to be a process vessel used to carry out a chemical reaction, which is one of the classic unit operations in chemical process analysis. The design of a chemical reactor deals with multiple aspects of chemical engineering. Chemical engineers design reactors to maximize net present value for the given reaction. Designers ensure that the reaction proceeds with the highest efficiency towards the desired output product, producing the highest yield of product while requiring the least amount of money to purchase and operate. Normal operating expenses include energy input, energy removal, raw material costs, labor, etc. Energy changes can come in the form of heating or cooling, pumping to increase pressure, frictional pressure loss or agitation.

A chemical plant is an industrial process plant that manufactures chemicals, usually on a large scale. The general objective of a chemical plant is to create new material wealth via the chemical or biological transformation and or separation of materials. Chemical plants use specialized equipment, units, and technology in the manufacturing process. Other kinds of plants, such as polymer, pharmaceutical, food, and some beverage production facilities, power plants, oil refineries or other refineries, natural gas processing and biochemical plants, water and wastewater treatment, and pollution control equipment use many technologies that have similarities to chemical plant technology such as fluid systems and chemical reactor systems. Some would consider an oil refinery or a pharmaceutical or polymer manufacturer to be effectively a chemical plant.
Supercritical fluid extraction (SFE) is the process of separating one component (the extractant) from another (the matrix) using supercritical fluids as the extracting solvent. Extraction is usually from a solid matrix, but can also be from liquids. SFE can be used as a sample preparation step for analytical purposes, or on a larger scale to either strip unwanted material from a product (e.g. decaffeination) or collect a desired product (e.g. essential oils). These essential oils can include limonene and other straight solvents. Carbon dioxide (CO2) is the most used supercritical fluid, sometimes modified by co-solvents such as ethanol or methanol. Extraction conditions for supercritical carbon dioxide are above the critical temperature of 31 °C and critical pressure of 74 bar. Addition of modifiers may slightly alter this. The discussion below will mainly refer to extraction with CO2, except where specified.
A batch reactor is a chemical reactor in which a non-continuous reaction is conducted, i.e., one where the reactants, products and solvent do not flow in or out of the vessel during the reaction until the target reaction conversion is achieved. By extension, the expression is somehow inappropriately used for other batch fluid processing operations that do not involve a chemical reaction, such as solids dissolution, product mixing, batch distillation, crystallization, and liquid/liquid extraction. In such cases, however, they may not be referred to as reactors but rather with a term specific to the function they perform.
In flow chemistry, also called reactor engineering, a chemical reaction is run in a continuously flowing stream rather than in batch production. In other words, pumps move fluid into a reactor, and where tubes join one another, the fluids contact one another. If these fluids are reactive, a reaction takes place. Flow chemistry is a well-established technique for use at a large scale when manufacturing large quantities of a given material. However, the term has only been coined recently for its application on a laboratory scale by chemists and describes small pilot plants, and lab-scale continuous plants. Often, microreactors are used.

A reaction calorimeter is a calorimeter that measures the amount of energy released (exothermic) or absorbed (endothermic) by a chemical reaction. These measurements provide a more accurate picture of such reactions.
Continuous reactors carry material as a flowing stream. Reactants are continuously fed into the reactor and emerge as continuous stream of product. Continuous reactors are used for a wide variety of chemical and biological processes within the food, chemical and pharmaceutical industries. A survey of the continuous reactor market will throw up a daunting variety of shapes and types of machine. Beneath this variation however lies a relatively small number of key design features which determine the capabilities of the reactor. When classifying continuous reactors, it can be more helpful to look at these design features rather than the whole system.
The Glossary of fuel cell terms lists the definitions of many terms used within the fuel cell industry. The terms in this fuel cell glossary may be used by fuel cell industry associations, in education material and fuel cell codes and standards to name but a few.
The three primary objectives of nuclear reactor safety systems as defined by the U.S. Nuclear Regulatory Commission are to shut down the reactor, maintain it in a shutdown condition and prevent the release of radioactive material.

Instrumentation is used to monitor and control the process plant in the oil, gas and petrochemical industries. Instrumentation ensures that the plant operates within defined parameters to produce materials of consistent quality and within the required specifications. It also ensures that the plant is operated safely and acts to correct out of tolerance operation and to automatically shut down the plant to prevent hazardous conditions from occurring. Instrumentation comprises sensor elements, signal transmitters, controllers, indicators and alarms, actuated valves, logic circuits and operator interfaces.
Boiling water reactor safety systems are nuclear safety systems constructed within boiling water reactors in order to prevent or mitigate environmental and health hazards in the event of accident or natural disaster.

The Fukushima Daiichi reactor, was 1 out of 4 reactors seriously effected during the Fukushima Daiichi nuclear disaster on 11 March 2011. Overall, the plant had 6 separate boiling water reactors originally designed by General Electric (GE), and maintained by the Tokyo Electric Power Company (TEPCO). At the time of the earthquake, Reactor 4 had been de-fueled while 5 and 6 were in cold shutdown for planned maintenance.
Workplace exposure monitoring is the monitoring of substances in a workplace that are chemical or biological hazards. It is performed in the context of workplace exposure assessment and risk assessment. Exposure monitoring analyzes hazardous substances in the air or on surfaces of a workplace, and is complementary to biomonitoring, which instead analyzes toxicants or their effects within workers.









