An emulsion is a mixture of two or more liquids that are normally immiscible owing to liquid-liquid phase separation. Emulsions are part of a more general class of two-phase systems of matter called colloids. Although the terms colloid and emulsion are sometimes used interchangeably, emulsion should be used when both phases, dispersed and continuous, are liquids. In an emulsion, one liquid is dispersed in the other. Examples of emulsions include vinaigrettes, homogenized milk, liquid biomolecular condensates, and some cutting fluids for metal working.

Butter is a dairy product made from the fat and protein components of churned cream. It is a semi-solid emulsion at room temperature, consisting of approximately 80% butterfat. It is used at room temperature as a spread, melted as a condiment, and used as a fat in baking, sauce-making, pan frying, and other cooking procedures.
Emulsion polymerization is a type of radical polymerization that usually starts with an emulsion incorporating water, monomer, and surfactant. The most common type of emulsion polymerization is an oil-in-water emulsion, in which droplets of monomer are emulsified in a continuous phase of water. Water-soluble polymers, such as certain polyvinyl alcohols or hydroxyethyl celluloses, can also be used to act as emulsifiers/stabilizers. The name "emulsion polymerization" is a misnomer that arises from a historical misconception. Rather than occurring in emulsion droplets, polymerization takes place in the latex/colloid particles that form spontaneously in the first few minutes of the process. These latex particles are typically 100 nm in size, and are made of many individual polymer chains. The particles are prevented from coagulating with each other because each particle is surrounded by the surfactant ('soap'); the charge on the surfactant repels other particles electrostatically. When water-soluble polymers are used as stabilizers instead of soap, the repulsion between particles arises because these water-soluble polymers form a 'hairy layer' around a particle that repels other particles, because pushing particles together would involve compressing these chains.

In chemistry, a suspension is a heterogeneous mixture of a fluid that contains solid particles sufficiently large for sedimentation. The particles may be visible to the naked eye, usually must be larger than one micrometer, and will eventually settle, although the mixture is only classified as a suspension when and while the particles have not settled out.

Flocculation, in the field of chemistry, is a process by which colloidal particles come out of suspension to sediment under the form of floc or flake, either spontaneously or due to the addition of a clarifying agent. The action differs from precipitation in that, prior to flocculation, colloids are merely suspended, under the form of a stable dispersion and are not truly dissolved in solution.

Churning is the process of shaking up cream or whole milk to make butter, usually using a butter churn. In Europe from the Middle Ages until the Industrial Revolution, a churn was usually as simple as a barrel with a plunger in it, moved by hand. These have mostly been replaced by mechanical churns.

A cream is a preparation usually for application to the skin. Creams for application to mucous membranes such as those of the rectum or vagina are also used. Creams may be considered pharmaceutical products as even cosmetic creams are based on techniques developed by pharmacy and unmedicated creams are highly used in a variety of skin conditions (dermatoses). The use of the finger tip unit concept may be helpful in guiding how much topical cream is required to cover different areas.
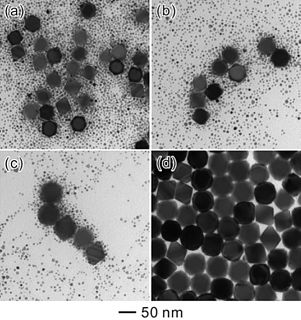
Ostwald ripening is a phenomenon observed in solid solutions or liquid sols that describes the change of an inhomogeneous structure over time, i.e., small crystals or sol particles dissolve, and redeposit onto larger crystals or sol particles.
A dispersion is a system in which distributed particles of one material are dispersed in a continuous phase of another material. The two phases may be in the same or different states of matter.
A miniemulsion is a special case of emulsion. A miniemulsion is obtained by shearing a mixture comprising two immiscible liquid phases, one or more surfactants and, possibly, one or more co-surfactants.

Water-in-water (W/W) emulsion is a system that consists of droplets of water-solvated molecules in another continuous aqueous solution; both the droplet and continuous phases contain different molecules that are entirely water-soluble. As such, when two entirely aqueous solutions containing different water-soluble molecules are mixed, water droplets containing predominantly one component are dispersed in water solution containing another component. Recently, such a water-in-water emulsion was demonstrated to exist and be stable from coalescence by the separation of different types of non-amphiphilic, but water-soluble molecular interactions. These molecular interactions include hydrogen bonding, pi stacking, and salt bridging. This w/w emulsion was generated when the different water-solvated molecular functional groups get segregated in an aqueous mixture consisting of polymer and liquid crystal molecules.

The ouzo effect is a milky oil-in-water emulsion that is formed when water is added to ouzo and other anise-flavored liqueurs and spirits, such as pastis, rakı, arak, sambuca and absinthe. Such emulsions occur with only minimal mixing and are highly stable.
A Pickering emulsion is an emulsion that is stabilized by solid particles which adsorb onto the interface between the water and oil phases. Typically, the emulsions are either water-in-oil or oil-in-water emulsions, but other more complex systems such as water-in-water, oil-in-oil, water-in-oil-in-water, and oil-in-water-in-oil also do exist. Pickering emulsions were named after S.U. Pickering, who described the phenomenon in 1907, although the effect was first recognized by Walter Ramsden in 1903.

An emulsion dispersion is thermoplastics or elastomers suspended in a liquid state by means of emulsifiers.
Meat emulsion is a two-phase system, with the dispersed phase consisting of either solid or liquid fat particles and the continuous phase being the water containing salts and dissolved, gelled and suspended proteins. Thus, they can be classified as oil-in-water emulsion. Meat emulsion is not a true emulsion since the two phases involved are not liquids and the fat droplets in a commercial emulsion are larger than 50 μm in diameter and thus do not conform to one of the requirement of a classical emulsion. Common examples of meat emulsions include bologna, frankfurters, sausages, and meatloaf.
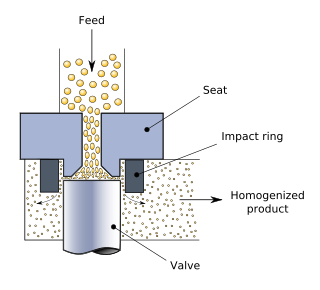
Homogenization or homogenisation is any of several processes used to make a mixture of two mutually non-soluble liquids the same throughout. This is achieved by turning one of the liquids into a state consisting of extremely small particles distributed uniformly throughout the other liquid. A typical example is the homogenization of milk, wherein the milk fat globules are reduced in size and dispersed uniformly through the rest of the milk.
Dispersions are unstable from the thermodynamic point of view; however, they can be kinetically stable over a large period of time, which determines their shelf life. This time span needs to be measured in order to ensure the best product quality to the final consumer. “Dispersion stability refers to the ability of a dispersion to resist change in its properties over time.” D.J. McClements.

Membrane emulsification (ME) is a relatively novel technique for producing all types of single and multiple emulsions for DDS, solid micro carriers for encapsulation of drug or nutrient, solder particles for surface-mount technology, mono dispersed polymer microspheres. Membrane emulsification was introduced by Nakashima and Shimizu in the late 1980s in Japan.
Emulsified Fuels are emulsions composed of water and a combustible liquid, either oil or a fuel. Emulsions are a particular example of a dispersion comprising a continuous and a dispersed phase. The most commonly used emulsion fuel is water-in-diesel emulsion. In the case of emulsions, both phases are the immiscible liquids, oil and water. Emulsion fuels can be either a microemulsion or an ordinary emulsion. The essential differences between the two are stability and particle size distribution. Microemulsions are isotropic whereas macroemulsions are prone to settling and changes in particle size over time. Both use surfactants and can be either water-in-oil, or oil-in-water or bicontinuous.
Macroemulsions are dispersed liquid-liquid, thermodynamically unstable systems with particle sizes ranging from 1 to 100 μm, which, most often, do not form spontaneously. Macroemulsions scatter light effectively and therefore appear milky, because their droplets are greater than a wavelength of light. They are part of a larger family of emulsions along with miniemulsions. As with all emulsions, one phase serves as the dispersing agent. It is often called the continuous or outer phase. The remaining phase(s) are disperse or inner phase(s), because the liquid droplets are finely distributed amongst the larger continuous phase droplets. This type of emulsion is thermodynamically unstable, but can be stabilized for a period of time with applications of kinetic energy. Surfactants are used to reduce the interfacial tension between the two phases, and induce macroemulsion stability for a useful amount of time. Emulsions can be stabilized otherwise with polymers, solid particles or proteins.










