An exon is any part of a gene that will encode a part of the final mature RNA produced by that gene after introns have been removed by RNA splicing. The term exon refers to both the DNA sequence within a gene and to the corresponding sequence in RNA transcripts. In RNA splicing, introns are removed and exons are covalently joined to one another as part of generating the mature messenger RNA. Just as the entire set of genes for a species constitutes the genome, the entire set of exons constitutes the exome.
Heat shock proteins (HSP) are a family of proteins that are produced by cells in response to exposure to stressful conditions. They were first described in relation to heat shock, but are now known to also be expressed during other stresses including exposure to cold, UV light and during wound healing or tissue remodeling. Many members of this group perform chaperone functions by stabilizing new proteins to ensure correct folding or by helping to refold proteins that were damaged by the cell stress. This increase in expression is transcriptionally regulated. The dramatic upregulation of the heat shock proteins is a key part of the heat shock response and is induced primarily by heat shock factor (HSF). HSPs are found in virtually all living organisms, from bacteria to humans.
The 5′ untranslated region is the region of an mRNA that is directly upstream from the initiation codon. This region is important for the regulation of translation of a transcript by differing mechanisms in viruses, prokaryotes and eukaryotes. While called untranslated, the 5′ UTR or a portion of it is sometimes translated into a protein product. This product can then regulate the translation of the main coding sequence of the mRNA. In many organisms, however, the 5′ UTR is completely untranslated, instead forming complex secondary structure to regulate translation. The 5′ UTR has been found to interact with proteins relating to metabolism; and proteins translate sequences within the 5′ UTR. In addition, this region has been involved in transcription regulation, such as the sex-lethal gene in Drosophila. Regulatory elements within 5′ UTRs have also been linked to mRNA export.
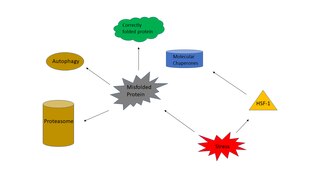
The heat shock response (HSR) is a cellular response that increases the number of molecular chaperones to combat the negative effects on proteins caused by stressors such as increased temperatures, oxidative stress, and heavy metals. In a normal cell, protein homeostasis (proteostasis) must be maintained because proteins are the main functional units of the cell. Proteins take on a defined configuration in order to gain functionality. If these structures are altered, critical processes could be affected, leading to cell damage or death. With the importance of proteins established, the heat shock response can be employed under stress to induce heat shock proteins (HSP), also known as molecular chaperones, that help prevent or reverse protein misfolding and provide an environment for proper folding.
Ribosome shunting is a mechanism of translation initiation in which ribosomes bypass, or "shunt over", parts of the 5' untranslated region to reach the start codon, enabling viruses to have more information than usual in an mRNA molecule. Some viral RNAs have been shown to use ribosome shunting as a more efficient form of translation during certain stages of viral life cycle or when translation initiation factors are scarce. Some viruses known to use this mechanism include adenovirus, Sendai virus, human papillomavirus, duck hepatitis B pararetrovirus, rice tungro bacilliform viruses, and cauliflower mosaic virus. In these viruses the ribosome is directly translocated from the upstream initiation complex to the start codon (AUG) without the need to unwind RNA secondary structures.
A structural gene is a gene that codes for any RNA or protein product other than a regulatory factor. A term derived from the lac operon, structural genes are typically viewed as those containing sequences of DNA corresponding to the amino acids of a protein that will be produced, as long as said protein does not function to regulate gene expression. Structural gene products include enzymes and structural proteins. Also encoded by structural genes are non-coding RNAs, such as rRNAs and tRNAs.
Cis-regulatory elements (CREs) are regions of non-coding DNA which regulate the transcription of neighboring genes. CREs are vital components of genetic regulatory networks, which in turn control morphogenesis, the development of anatomy, and other aspects of embryonic development, studied in evolutionary developmental biology.
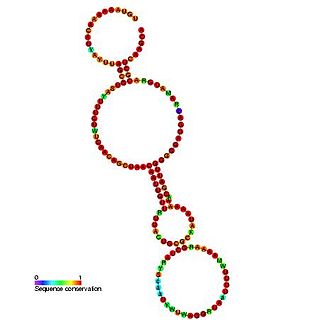
The PrfA thermoregulator UTR is an RNA thermometer found in the 5' UTR of the prfA gene. In Listeria monocytogenes, virulence genes are maximally expressed at 37 °C but are almost silent at 30 °C. The genes are controlled by PrfA, a transcriptional activator whose expression is thermoregulated. It has been shown that the untranslated mRNA (UTR) preceding prfA, forms a secondary structure, which masks the ribosome binding region. It is thought that at 37 °C, the hairpin structure 'melts' and the SD sequence is unmasked.

The repression of heat shock gene expression (ROSE) element is an RNA element found in the 5' UTR of some heat shock protein's mRNAs. The ROSE element is an RNA thermometer that negatively regulates heat shock gene expression. The secondary structure is thought to be altered by temperature, thus it is an RNA thermometer. This structure blocks access to the ribosome binding site at normal temperatures. During heat shock however, the structure changes freeing the ribosome binding site and allowing expression to occur.

The Hsp90 cis regulatory element is an RNA element found in the 5' UTR of the Drosophila hsp90 mRNA. It is required for increased translational efficiency during the heat shock response.

In molecular genetics, an untranslated region refers to either of two sections, one on each side of a coding sequence on a strand of mRNA. If it is found on the 5' side, it is called the 5' UTR, or if it is found on the 3' side, it is called the 3' UTR. mRNA is RNA that carries information from DNA to the ribosome, the site of protein synthesis (translation) within a cell. The mRNA is initially transcribed from the corresponding DNA sequence and then translated into protein. However, several regions of the mRNA are usually not translated into protein, including the 5' and 3' UTRs.

Heat shock 70 kDa protein 1L is a protein that in humans is encoded by the HSPA1L gene on chromosome 6. As a member of the heat shock protein 70 (Hsp70) family and a chaperone protein, it facilitates the proper folding of newly translated and misfolded proteins, as well as stabilize or degrade mutant proteins. Its functions contribute to biological processes including signal transduction, apoptosis, protein homeostasis, and cell growth and differentiation. It has been associated with an extensive number of cancers, neurodegenerative diseases, cell senescence and aging, and Graft-versus-host disease.

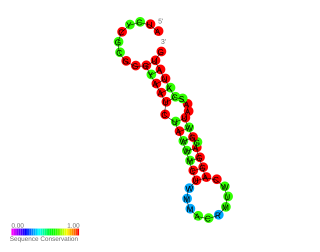
FourU thermometers are a class of non-coding RNA thermometers found in Salmonella. They are named 'FourU' due to the four highly conserved uridine nucleotides found directly opposite the Shine-Dalgarno sequence on hairpin II (pictured). RNA thermometers such as FourU control regulation of temperature via heat shock proteins in many prokaryotes. FourU thermometers are relatively small RNA molecules, only 57 nucleotides in length, and have a simple two-hairpin structure.
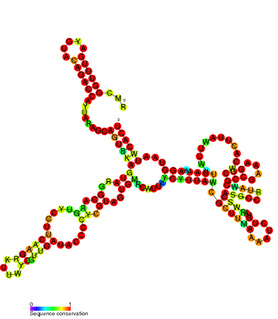
cspA mRNA 5' UTR is the untranslated region of the cspA gene, which is important in the cold shock response in Enterobacteriales such as E. coli. The 5' UTR element acts as an RNA thermometer, regulating the expression of cspA in response to temperature. By regulating temperature, cspA proteins carry out the vital function of homeostasis.
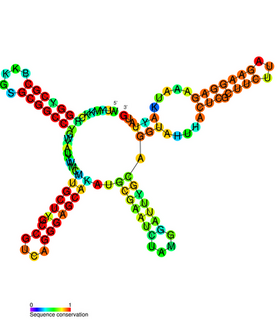
The IbpB thermometer is an RNA thermometer element found in the ibpAB operon. The operon contains two heat-shock genes, encoding inclusion body binding proteins A and B (IbpA/B), and is the most drastically upregulated operon under heat-shock in Escherichia coli.

RNA thermometers regulate gene expression in response to temperature allowing pathogens like Yersinia to switch on silent genes after entering the host organism. Usually, RNA thermometers are located in the 5'UTR, but an intergenic RNA thermometer was found in Yersinia pseudotuberculosis. The LcrFRNA thermometer together with the termo-labile YmoA protein activates synthesis of the most crucial virulence activator LcrF (VirF). The RNA thermosensor sequence is 100% identical in all human pathogenic Yersinia species.

Smaug is a RNA binding protein in Drosophila that helps in maternal to zygotic transition (MZT). The protein is named after the fictional character Smaug, the dragon in J.R.R. Tolkien's 1937 novel The Hobbit. The MZT ends with the midblastula transition (MBT), which is defined as the first developmental event in Drosophila that depends on zygotic mRNA. In Drosophila, the initial developmental events are controlled by maternal mRNAs like Hsp83, nanos, string, Pgc, and cyclin B mRNA. Degradation of these mRNAs, which is expected to terminate maternal control and enable zygotic control of embryogenesis, happens at interphase of nuclear division cycle 14. During this transition smaug protein targets the maternal mRNA for destruction using miRs. Thus activating the zygotic genes. Smaug is expected to play a role in expression of three miRNAs – miR-3, miR-6, miR-309 and miR-286 during MZT in Drosophila. Among them smaug dependent expression of miR-309 is needed for destabilization of 410 maternal mRNA. In smaug mutants almost 85% of maternal mRNA is found to be stable. Smaug also bind to 3′ untranslated region (UTR) elements known as SMG response elements (SREs) on nanos mRNA and represses its expression by recruiting a protein called Cup(an eIF4E-binding protein that blocks the binding of eIF4G to eIF4E). There after it recruits deadenylation complex CCR4-NOT on to the nanos mRNA which leads to deadenylation and subsequent decay of the mRNA. It is also found to be involved in degradation and repression of maternal Hsp83 mRNA by recruiting CCR4/POP2/NOT deadenylase to the mRNA.
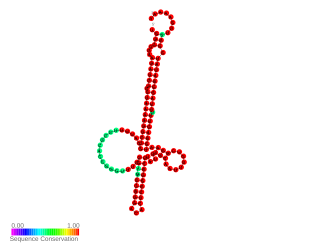
RNA thermometers (RNATs) regulate gene expression in response to temperature, allowing pathogens such as Neisseria meningitidis to switch on silent genes after entering the host organism. However the temperature for expression of Neisseria virulence-associated traits is 42 °C while other bacterial pathogen RNATs require 37 °C. This is probably because N. meningitidis is an obligate commensal of the human nasopharynx and becomes pathogenic during inflammation due to viral infection. Three independent RNA thermosensors were identified in the 5'UTRs of genes needed for: capsule biosynthesis (cssA), the expression of factor H binding protein (fHbp) and sialylation of lipopolysaccharide, which is essential for bacterial resistance against immune killing (lst). The very different nucleotide sequence and predicted inhibitory structures of the three RNATs indicate that they have evolved independently.