
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residues. Proteins perform a vast array of functions within organisms, including catalysing metabolic reactions, DNA replication, responding to stimuli, providing structure to cells and organisms, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific 3D structure that determines its activity.

A zinc finger is a small protein structural motif that is characterized by the coordination of one or more zinc ions (Zn2+) which stabilizes the fold. It was originally coined to describe the finger-like appearance of a hypothesized structure from the African clawed frog (Xenopus laevis) transcription factor IIIA. However, it has been found to encompass a wide variety of differing protein structures in eukaryotic cells. Xenopus laevis TFIIIA was originally demonstrated to contain zinc and require the metal for function in 1983, the first such reported zinc requirement for a gene regulatory protein followed soon thereafter by the Krüppel factor in Drosophila. It often appears as a metal-binding domain in multi-domain proteins.

A peptidomimetic is a small protein-like chain designed to mimic a peptide. They typically arise either from modification of an existing peptide, or by designing similar systems that mimic peptides, such as peptoids and β-peptides. Irrespective of the approach, the altered chemical structure is designed to advantageously adjust the molecular properties such as stability or biological activity. This can have a role in the development of drug-like compounds from existing peptides. Peptidomimetics can be prepared by cyclization of linear peptides or coupling of stable unnatural amino acids. These modifications involve changes to the peptide that will not occur naturally. Unnatural amino acids can be generated from their native analogs via modifications such as amine alkylation, side chain substitution, structural bond extension cyclization, and isosteric replacements within the amino acid backbone. Based on their similarity with the precursor peptide, peptidomimetics can be grouped into four classes where A features the most and D the least similarities. Classes A and B involve peptide-like scaffolds, while classes C and D include small molecules.

Phage display is a laboratory technique for the study of protein–protein, protein–peptide, and protein–DNA interactions that uses bacteriophages to connect proteins with the genetic information that encodes them. In this technique, a gene encoding a protein of interest is inserted into a phage coat protein gene, causing the phage to "display" the protein on its outside while containing the gene for the protein on its inside, resulting in a connection between genotype and phenotype. The proteins that the phages are displaying can then be screened against other proteins, peptides or DNA sequences, in order to detect interaction between the displayed protein and those of other molecules. In this way, large libraries of proteins can be screened and amplified in a process called in vitro selection, which is analogous to natural selection.
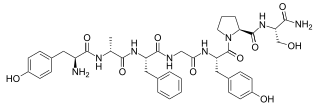
Dermorphin is a hepta-peptide first isolated from the skin of South American frogs belonging to the genus Phyllomedusa. The peptide is a natural opioid that binds as an agonist with high potency and selectivity to mu opioid receptors. Dermorphin is about 30–40 times more potent than morphine, but theoretically may be less likely to produce drug tolerance and addiction due to its high potency. The amino acid sequence of dermorphin is H-Tyr-D-Ala-Phe-Gly-Tyr-Pro-Ser-NH2.
Ribosome display is a technique used to perform in vitro protein evolution to create proteins that can bind to a desired ligand. The process results in translated proteins that are associated with their mRNA progenitor which is used, as a complex, to bind to an immobilized ligand in a selection step. The mRNA-protein hybrids that bind well are then reverse transcribed to cDNA and their sequence amplified via PCR. The result is a nucleotide sequence that can be used to create tightly binding proteins.

Aptamers are short sequences of artificial DNA, RNA, XNA, or peptide that bind a specific target molecule, or family of target molecules. They exhibit a range of affinities, with variable levels of off-target binding and are sometimes classified as chemical antibodies. Aptamers and antibodies can be used in many of the same applications, but the nucleic acid-based structure of aptamers, which are mostly oligonucleotides, is very different from the amino acid-based structure of antibodies, which are proteins. This difference can make aptamers a better choice than antibodies for some purposes.

In immunology, epitope mapping is the process of experimentally identifying the binding site, or epitope, of an antibody on its target antigen. Identification and characterization of antibody binding sites aid in the discovery and development of new therapeutics, vaccines, and diagnostics. Epitope characterization can also help elucidate the binding mechanism of an antibody and can strengthen intellectual property (patent) protection. Experimental epitope mapping data can be incorporated into robust algorithms to facilitate in silico prediction of B-cell epitopes based on sequence and/or structural data.

The trp operon is a group of genes that are transcribed together, encoding the enzymes that produce the amino acid tryptophan in bacteria. The trp operon was first characterized in Escherichia coli, and it has since been discovered in many other bacteria. The operon is regulated so that, when tryptophan is present in the environment, the genes for tryptophan synthesis are repressed.

mRNA display is a display technique used for in vitro protein, and/or peptide evolution to create molecules that can bind to a desired target. The process results in translated peptides or proteins that are associated with their mRNA progenitor via a puromycin linkage. The complex then binds to an immobilized target in a selection step. The mRNA-protein fusions that bind well are then reverse transcribed to cDNA and their sequence amplified via a polymerase chain reaction. The result is a nucleotide sequence that encodes a peptide with high affinity for the molecule of interest.

Lysins, also known as endolysins or murein hydrolases, are hydrolytic enzymes produced by bacteriophages in order to cleave the host's cell wall during the final stage of the lytic cycle. Lysins are highly evolved enzymes that are able to target one of the five bonds in peptidoglycan (murein), the main component of bacterial cell walls, which allows the release of progeny virions from the lysed cell. Cell-wall-containing Archaea are also lysed by specialized pseudomurein-cleaving lysins, while most archaeal viruses employ alternative mechanisms. Similarly, not all bacteriophages synthesize lysins: some small single-stranded DNA and RNA phages produce membrane proteins that activate the host's autolytic mechanisms such as autolysins.
Zinc finger protein chimera are chimeric proteins composed of a DNA-binding zinc finger protein domain and another domain through which the protein exerts its effect. The effector domain may be a transcriptional activator (A) or repressor (R), a methylation domain (M) or a nuclease (N).
Imperatoxin I (IpTx) is a peptide toxin derived from the venom of the African scorpion Pandinus imperator.

Racemic crystallography is a technique used in structural biology where crystals of a protein molecule are developed from an equimolar mixture of an L-protein molecule of natural chirality and its D-protein mirror image. L-protein molecules consist of 'left-handed' L-amino acids and the achiral amino acid glycine, whereas the mirror image D-protein molecules consist of 'right-handed' D-amino acids and glycine. Typically, both the L-protein and the D-protein are prepared by total chemical synthesis.
DNA-encoded chemical libraries (DECL) is a technology for the synthesis and screening on an unprecedented scale of collections of small molecule compounds. DECL is used in medicinal chemistry to bridge the fields of combinatorial chemistry and molecular biology. The aim of DECL technology is to accelerate the drug discovery process and in particular early phase discovery activities such as target validation and hit identification.
Affibody molecules are small, robust proteins engineered to bind to a large number of target proteins or peptides with high affinity, imitating monoclonal antibodies, and are therefore a member of the family of antibody mimetics. Affibody molecules are used in biochemical research and are being developed as potential new biopharmaceutical drugs. These molecules can be used for molecular recognition in diagnostic and therapeutic applications.
DARPins are genetically engineered antibody mimetic proteins typically exhibiting highly specific and high-affinity target protein binding. They are derived from natural ankyrin repeat proteins, one of the most common classes of binding proteins in nature, which are responsible for diverse functions such as cell signaling, regulation and structural integrity of the cell. DARPins consist of at least three, repeat motifs or modules, of which the most N- and the most C-terminal modules are referred to as "caps", since they shield the hydrophobic core of the protein. The number of internal modules is indicated as number while the caps are indicated with "N" or "C", respectively. The molecular mass of e.g. 14 or 18 kDa (kilodaltons) for four- (N2C) or five- (N3C) repeat DARPins is rather small for a biologic.
Avimers are artificial proteins that are able to specifically bind to certain antigens via multiple binding sites. Since they are not structurally related to antibodies, they are classified as a type of antibody mimetic. Avimers have been developed by the biotechnology company Avidia, now part of Amgen, as potential new pharmaceutical drugs.
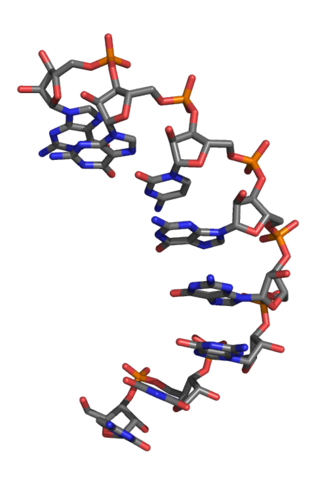
An L-ribonucleic acid aptamer is an RNA-like molecule built from L-ribose units. It is an artificial oligonucleotide named for being a mirror image of natural oligonucleotides. L-RNA aptamers are a form of aptamers. Due to their L-nucleotides, they are highly resistant to degradation by nucleases. L-RNA aptamers are considered potential drugs and are currently being tested in clinical trials.

A collagen hybridizing peptide (CHP) is a synthetic peptide sequence with typically 6 to 10 repeating units of the Gly-Xaa-Yaa amino acid triplet, which mimics the hallmark sequence of natural collagens. A CHP peptide usually possesses a high content of proline and hydroxyproline in the Xaa and Yaa positions, which confers it a strong propensity to form the collagen's unique triple helix conformation. In the single-stranded (monomeric) status, the peptide can recognize denatured collagen strands in tissues by forming a hybridized triple helix with the collagen strands. This occurs via the triple helical chain assembly and inter-chain hydrogen bonding, in a manner similar to primers binding to melted DNA strands during PCR. The binding does not depend on a specific sequence or epitope on collagen, enabling CHPs to target denatured collagen chains of different types.















