Related Research Articles

Cardioversion is a medical procedure by which an abnormally fast heart rate (tachycardia) or other cardiac arrhythmia is converted to a normal rhythm using electricity or drugs.
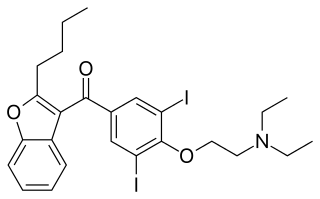
Antiarrhythmic agents, also known as cardiac dysrhythmia medications, are a class of drugs that are used to suppress abnormally fast rhythms (tachycardias), such as atrial fibrillation, supraventricular tachycardia and ventricular tachycardia.
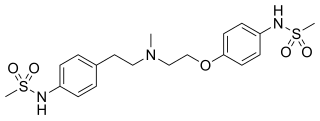
Dofetilide is a class III antiarrhythmic agent. It is marketed under the trade name Tikosyn by Pfizer, and is available in the United States in capsules containing 125, 250, and 500 μg of dofetilide. It is not available in Europe or Australia.
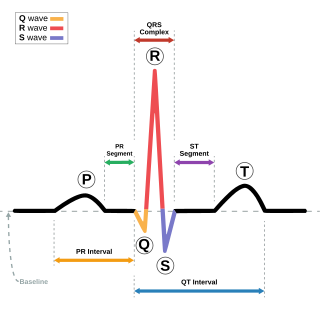
Short QT syndrome (SQT) is a very rare genetic disease of the electrical system of the heart, and is associated with an increased risk of abnormal heart rhythms and sudden cardiac death. The syndrome gets its name from a characteristic feature seen on an electrocardiogram (ECG) – a shortening of the QT interval. It is caused by mutations in genes encoding ion channels that shorten the cardiac action potential, and appears to be inherited in an autosomal dominant pattern. The condition is diagnosed using a 12-lead ECG. Short QT syndrome can be treated using an implantable cardioverter-defibrillator or medications including quinidine. Short QT syndrome was first described in 2000, and the first genetic mutation associated with the condition was identified in 2004.

Quinidine is a class IA antiarrhythmic agent used to treat heart rhythm disturbances. It is a diastereomer of antimalarial agent quinine, originally derived from the bark of the cinchona tree. The drug causes increased action potential duration, as well as a prolonged QT interval. As of 2019, its IV formulation is no longer being manufactured for use in the United States.

Sotalol, sold under the brand name Betapace among others, is a medication used to treat and prevent abnormal heart rhythms. Evidence does not support a decreased risk of death with long term use. It is taken by mouth or given by injection into a vein.

Catheter ablation is a procedure that uses radio-frequency energy or other sources to terminate or modify a faulty electrical pathway from sections of the heart of those who are prone to developing cardiac arrhythmias such as atrial fibrillation, atrial flutter and Wolff-Parkinson-White syndrome. If not controlled, such arrhythmias increase the risk of ventricular fibrillation and sudden cardiac arrest. The ablation procedure can be classified by energy source: radiofrequency ablation and cryoablation.
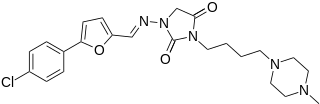
Azimilide is a class ΙΙΙ antiarrhythmic drug. The agents from this heterogeneous group have an effect on the repolarization, they prolong the duration of the action potential and the refractory period. Also they slow down the spontaneous discharge frequency of automatic pacemakers by depressing the slope of diastolic depolarization. They shift the threshold towards zero or hyperpolarize the membrane potential. Although each agent has its own properties and will have thus a different function.

Dronedarone, sold under the brand name Multaq, is a class III antiarrhythmic medication developed by Sanofi-Aventis. It was approved by the US Food and Drug Administration (FDA) in July 2009. Besides being indicated in arrhythmias, it was recommended as an alternative to amiodarone for the treatment of atrial fibrillation and atrial flutter in people whose hearts have either returned to normal rhythm or who undergo drug therapy or electric shock treatment i.e. direct current cardioversion (DCCV) to maintain normal rhythm. It is a class III antiarrhythmic drug. The FDA label includes a claim for reducing hospitalization, but not for reducing mortality, as a reduction in mortality was not demonstrated in the clinical development program. A trial of the drug in heart failure was stopped as an interim analysis showed a possible increase in heart failure deaths, in people with moderate to severe congestive heart failure.

The Libin Cardiovascular Institute is an entity of Alberta Health Services and the University of Calgary. It connects all cardiovascular research, education and patient care in Southern Alberta, serving a population of about two million. Its more than 1,500 members include physicians, clinicians and other health professionals, researchers and trainees.

Pilsicainide (INN) is an antiarrhythmic agent. It is marketed in Japan as サンリズム (Sunrythm). It was developed by Suntory Holdings Limited and first released in 1991. The JAN applies to the hydrochloride salt, pilsicainide hydrochloride.

Atrial fibrillation is an abnormal heart rhythm (arrhythmia) characterized by rapid and irregular beating of the atrial chambers of the heart. It often begins as short periods of abnormal beating, which become longer or continuous over time. It may also start as other forms of arrhythmia such as atrial flutter that then transform into AF.

Arrhythmias, also known as cardiac arrhythmias, are irregularities in the heartbeat, including when it is too fast or too slow. A resting heart rate that is too fast – above 100 beats per minute in adults – is called tachycardia, and a resting heart rate that is too slow – below 60 beats per minute – is called bradycardia. Some types of arrhythmias have no symptoms. Symptoms, when present, may include palpitations or feeling a pause between heartbeats. In more serious cases, there may be lightheadedness, passing out, shortness of breath, chest pain, or decreased level of consciousness. While most cases of arrhythmia are not serious, some predispose a person to complications such as stroke or heart failure. Others may result in sudden death.

Celivarone is an experimental drug being tested for use in pharmacological antiarrhythmic therapy. Cardiac arrhythmia is any abnormality in the electrical activity of the heart. Arrhythmias range from mild to severe, sometimes causing symptoms like palpitations, dizziness, fainting, and even death. They can manifest as slow (bradycardia) or fast (tachycardia) heart rate, and may have a regular or irregular rhythm.

Rotigaptide (ZP-123) is a drug under clinical investigation for the treatment of cardiac arrhythmias – specifically atrial fibrillation. It is a peptide analog that has been shown to increase gap junction intercellular conductance in cardiac muscle cells. Gap junctions are protein channels that are responsible for conducting electrical impulses between cells in the heart to maintain normal rhythm. Gap junction modulation is a promising and novel mechanism of action for the treatment of cardiovascular disorders. Its peptide sequence is Ac-D-Tyr-D-Pro-D-Hyp-Gly-D-Ala-Gly-NH2.

Budiodarone (ATI-2042) is an antiarrhythmic agent and chemical analog of amiodarone that is currently being studied in clinical trials. Amiodarone is considered the most effective antiarrhythmic drug available, but its adverse side effects, including hepatic, pulmonary and thyroid toxicity as well as multiple drug interactions, are discouraging its use. Budiodarone only differs in structure from amiodarone through the presence of a sec-butyl acetate side chain at position 2 of the benzofuran moiety. This side chain allows for budiodarone to have a shorter half-life in the body than amiodarone which allows it to have a faster onset of action and metabolism while still maintaining similar electrophysiological activity. The faster metabolism of budiodarone allows for fewer adverse side effects than amiodarone principally due to decreased levels of toxicity in the body.

HBI-3000 is an experimental drug candidate that is currently in phase II of human clinical trials as an antiarrhythmic agent. Clinical investigation will test the safety and efficacy of HBI-3000 as a treatment for both atrial and ventricular arrhythmias.
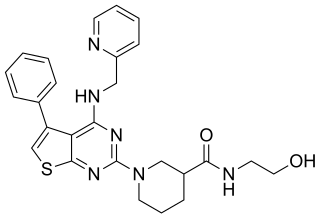
XEN-D0101 is an experimental drug that was developed to treat atrial fibrillation. Xention, a biopharmaceutical company based in Cambridge, England, created XEN-D0101 along with other ion channel-modulating drugs. XEN-D0101 is a selective antagonist of the voltage-gated potassium channel Kv1.5. Atrial fibrillation is the main focus of Xention’s drug development, as it is the most common cardiac arrhythmia seen in patients.
Yaariv Khaykin is a Canadian cardiologist and a clinical researcher in the area of electrophysiology. He is the director of the Newmarket Electrophysiology Research Group at the Southlake Regional Health Centre. He has published research into complex ablation and pioneered cardiac ablation methods.

Günter Breithardt is a German physician, cardiologist and emeritus university professor. He is known for his research in the field of rhythmology, especially the diagnosis and pharmacological and non-pharmacological therapy of cardiac arrhythmias and acute cardiac death, in particular the identification of arrhythmia-triggering gene mutations. For 21 years he headed the Medical Clinic and Polyclinic C at Münster University Hospital. A number of his academic students hold university management and chief physician positions.
References
- ↑ International Experts Advisory Committee Archived 2008-06-07 at the Wayback Machine - Libin Cardiovascular Institute of Alberta
- ↑ Press Release Archived 2012-09-22 at the Wayback Machine - Lecture to Seniors
- ↑ May 12, 2007 edition of Heart Rhythm Daily Archived May 19, 2011, at the Wayback Machine - a publication of the Heart Rhythm Society
- ↑ Wyse D, Waldo A, DiMarco J, Domanski M, Rosenberg Y, Schron E, Kellen J, Greene H, Mickel M, Dalquist J, Corley S (2002). "A comparison of rate control and rhythm control in patients with atrial fibrillation". N Engl J Med. 347 (23): 1825–33. doi: 10.1056/NEJMoa021328 . PMID 12466506.
- ↑ Wyse DG, Morganroth J, Ledingham R, Denes P, Hallstrom A, Mitchell LB, Epstein AE, Woosley RL, Capone R (1994). "New insights into the definition and meaning of proarrhythmia during initiation of antiarrhythmic drug therapy from the Cardiac Arrhythmia Suppression Trial and its pilot study. The CAST and CAPS Investigators". J Am Coll Cardiol. 23 (5): 1130–40. doi:10.1016/0735-1097(94)90601-7. PMID 8144779.
- ↑ "Dr. D. George Wyse - antiarrhythmic drugs". Archived from the original on August 23, 2007. Retrieved 2008-08-27.
- ↑ Citation Archived 2011-06-06 at the Wayback Machine - Distinguished Alumni
- ↑ Distinguished Scientist Archived 2008-09-17 at the Wayback Machine - Heart Rhythm Society
- ↑ Citation Archived 2016-09-17 at the Wayback Machine - Top 40 Alumni
- ↑ Citation Archived 2009-02-03 at the Wayback Machine - CCS Annual Achievement Award
- ↑ "George Wyse to speak at 2017 Lecture of a Lifetime". 19 April 2017.