
Thalidomide, sold under the brand name Immunoprin, among others, is an immunomodulatory drug and the prototype of the thalidomide class of drugs. Today, thalidomide is used mainly as a treatment of certain cancers and of a complication of leprosy.
POEMS syndrome is a rare paraneoplastic syndrome caused by a clone of aberrant plasma cells. The name POEMS is an acronym for some of the disease's major signs and symptoms, as is PEP.

Lenalidomide is a derivative of thalidomide approved in the United States in 2005.
Moses Judah Folkman was an American medical scientist best known for his research on tumor angiogenesis, the process by which a tumor attracts blood vessels to nourish itself and sustain its existence. He founded the field of angiogenesis research, which has led to the discovery of a number of therapies based on inhibiting or stimulating neovascularization.

Monoclonal gammopathy of undetermined significance (MGUS) is a plasma cell dyscrasia in which plasma cells or other types of antibody-producing cells secret a myeloma protein, i.e. an abnormal antibody, into the blood; this abnormal protein is usually found and in the blood and/or urine during standard laboratory blood or urine tests. MGUS resembles multiple myeloma and similar diseases, but the levels of antibodies are lower, the number of plasma cells in the bone marrow is lower, and it rarely has symptoms or major problems. However, since MGUS can lead to multiple myeloma which develops at the rate of about 1.5% a year, yearly monitoring is recommended.

A Bence Jones protein is a monoclonal globulin protein or immunoglobulin light chain found in the urine, with a molecular weight of 22-24 kDa. Detection of Bence Jones protein may be suggestive of multiple myeloma or Waldenström's macroglobulinemia.

Melphalan is a chemotherapy drug belonging to the class of nitrogen mustard alkylating agents.
Waldenström's macroglobulinemia (WM), also known as lymphoplasmacytic lymphoma, is a type of cancer affecting two types of B cells, lymphoplasmacytoid cells and plasma cells. Both cell types are white blood cells. WM is characterized by having high levels of a circulating antibody, immunoglobulin M (IgM), which is made and secreted by the cells involved in the disease. WM is an "indolent lymphoma" and a type of lymphoproliferative disease which shares clinical characteristics with the indolent non-Hodgkin lymphomas. WM is commonly classified as a form of plasma cell dyscrasia. Similar to other plasma cell dyscrasias that, for example, lead to multiple myeloma, WM is commonly preceded by two clinically asymptomatic but progressively more pre-malignant phases, IgM monoclonal gammopathy of undetermined significance and smoldering Waldenström's macroglobulinemia. The WM spectrum of dysplasias differs from other spectrums of plasma cell dyscrasias in that it involves not only aberrant plasma cells but also aberrant lymphoplasmacytoid cells and that it involves IgM while other plasma dyscrasias involve other antibody isoforms.

Plasma cell leukemia (PCL) is a plasma cell dyscrasia, i.e. a disease involving the malignant degeneration of a subtype of white blood cells called plasma cells. It is the terminal stage and most aggressive form of these dyscrasias, constituting 2% to 4% of all cases of plasma cell malignancies. PCL may present as primary plasma cell leukemia, i.e. in patients without prior history of a plasma cell dyscrasia or as secondary plasma cell dyscrasia, i.e. in patients previously diagnosed with a history of its predecessor dyscrasia, multiple myeloma. The two forms of PCL appear to be at least partially distinct from each other. In all cases, however, PCL is an extremely serious, life-threatening, and therapeutically challenging disease.

Pomalidomide is a derivative of thalidomide marketed by Celgene. It is anti-angiogenic and also acts as an immunomodulator.
Chromonychia is an abnormality in color of the substance or surface of the nail plate or subungual tissues.

Professor Jacob Sheskin, sometimes known as Sheskin Jacob was an Israeli physician best known for his 1964 serendipitous discovery that thalidomide can be used as a treatment for leprosy at Hadassah University in Jerusalem.

Carfilzomib is an anti-cancer drug acting as a selective proteasome inhibitor. Chemically, it is a tetrapeptide epoxyketone and an analog of epoxomicin.

The development of analogs of thalidomide was precipitated by the discovery of the anti-angiogenic and anti-inflammatory properties of the drug yielding a new way of fighting cancer as well as some inflammatory diseases after it had been banned in 1961. The problems with thalidomide included; teratogenic side effects, high incidence of other adverse reactions, poor solubility in water and poor absorption from the intestines.

Immunomodulatory imide drugs (IMiDs) are a class of immunomodulatory drugs containing an imide group. The IMiD class includes thalidomide and its analogues.
Smouldering myeloma, also known as smoldering myeloma, smoldering multiple myeloma, indolent myeloma or asymptomatic myeloma, is a disease classified as intermediate in a spectrum of step-wise progressive diseases termed plasma cell dyscrasias. In this spectrum of diseases, a clone of plasma cells secreting monoclonal paraprotein causes the relatively benign disease of monoclonal gammopathy of undetermined significance. This clone proliferates and may slowly evolve into more aggressive sub-clones that cause smouldering multiple myeloma. Further and more rapid evolution causes the overtly malignant stage of multiple myeloma and can subsequently lead to the extremely malignant stage of secondary plasma cell leukemia. Thus, some patients with smouldering myeloma progress to multiple myeloma and plasma cell leukemia. Smouldering myeloma, however, is not a malignant disease. It is characterised as a pre-malignant disease that lacks symptoms but is associated with bone marrow biopsy showing the presence of an abnormal number of clonal myeloma cells, blood and/or urine containing a myeloma protein, and a significant risk of developing into a malignant disease.

CC-1088 is a thalidomide analogue inhibitor of phosphodiesterase 4 that was being developed up to 2005 by Celgene Corp., for treating of inflammatory diseases and myelodysplastic syndromes. Apremilast (CC-10004) was found to be a preferable.
Sequential high-dose chemotherapy is a chemotherapy regimen consisting of several sequential monochemotherapies with only one chemotherapeutic agent per course. The idea behind this approach is that when using single-agent chemotherapy, the doctor can easily escalate the dose of the drug to the maximum tolerable dose by the patient, avoiding additive hematological toxicity from chemotherapeutic combinations, and thus improving efficacy. It is mostly used as consolidation therapy for relapsed or refractory lymphomas and relapsed or refractory Hodgkin disease, after DHAP induction. There is also an ongoing trial of this approach in multiple myeloma.
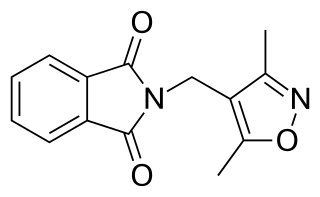
DIMP, or N-(3,5-dimethyl-4-isoxazolylmethyl)phthalimide, is a nonsteroidal antiandrogen (NSAA) structurally related to thalidomide that was first described in 1973 and was never marketed. Along with flutamide, it was one of the earliest NSAAs to be discovered, and for this reason, has been described as a "classical" NSAA. The drug is a selective, competitive, silent antagonist of the AR, although it is described as an "only relatively weak competitor". Its relative binding affinity for the androgen receptor is about 2.6% of that of metribolone. DIMP possesses no androgenic, estrogenic, progestogenic, or antigonadotropic activity, but it does reverse the antigonadotropic effects of testosterone, indicating that, like other pure AR antagonists, it is progonadotropic.













