Related Research Articles

A catalytic converter is an exhaust emission control device which converts toxic gases and pollutants in exhaust gas from an internal combustion engine into less-toxic pollutants by catalyzing a redox reaction. Catalytic converters are usually used with internal combustion engines fueled by gasoline or diesel, including lean-burn engines, and sometimes on kerosene heaters and stoves.
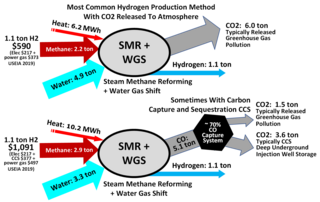
Steam reforming or steam methane reforming (SMR) is a method for producing syngas (hydrogen and carbon monoxide) by reaction of hydrocarbons with water. Commonly natural gas is the feedstock. The main purpose of this technology is often hydrogen production, although syngas has multiple other uses such as production of ammonia or methanol. The reaction is represented by this equilibrium:

Selective catalytic reduction (SCR) means converting nitrogen oxides, also referred to as NO
x with the aid of a catalyst into diatomic nitrogen, and water. A reductant, typically anhydrous ammonia, aqueous ammonia, or a urea solution, is added to a stream of flue or exhaust gas and is reacted onto a catalyst. As the reaction drives toward completion, nitrogen, and carbon dioxide, in the case of urea use, are produced.
A nitrogen oxide sensor or NOx sensor is typically a high-temperature device built to detect nitrogen oxides in combustion environments such as an automobile, truck tailpipe or smokestack.

Diesel exhaust fluid is a liquid used to reduce the amount of air pollution created by a diesel engine. Specifically, DEF is an aqueous urea solution made with 32.5% urea and 67.5% deionized water. DEF is consumed in a selective catalytic reduction (SCR) that lowers the concentration of nitrogen oxides in the diesel exhaust emissions from a diesel engine.
In atmospheric chemistry, NOx is shorthand for nitric oxide and nitrogen dioxide, the nitrogen oxides that are most relevant for air pollution. These gases contribute to the formation of smog and acid rain, as well as affecting tropospheric ozone.
A NOx adsorber or NOx trap (also called Lean NOx trap, abbr. LNT) is a device that is used to reduce oxides of nitrogen (NO and NO2) emissions from a lean burn internal combustion engine by means of adsorption.
Selective non-catalytic reduction (SNCR) is a method to lessen nitrogen oxide emissions in conventional power plants that burn biomass, waste and coal. The process involves injecting either ammonia or urea into the firebox of the boiler at a location where the flue gas is between 1,400 and 2,000 °F (760 and 1,090 °C) to react with the nitrogen oxides formed in the combustion process. The resulting product of the chemical redox reaction is molecular nitrogen (N2), carbon dioxide (CO2), and water (H2O).

William C. Pfefferle was an American scientist and inventor.

Neal Russell Amundson was an American chemical engineer and applied mathematician. He was the chair of the department of chemical engineering at the University of Minnesota for over 25 years. Later, he was the Cullen Professor of Chemical & Biomolecular Engineering and Mathematics at the University of Houston. Amundson was considered one of the most prominent chemical engineering educators and researchers in the United States. The Chemical Engineering and Materials Science building at the University of Minnesota-Twin Cities bears his name.
Reactive flash volatilization (RFV) is a chemical process that rapidly converts nonvolatile solids and liquids to volatile compounds by thermal decomposition for integration with catalytic chemistries.

Lanny D. Schmidt was an American chemist, inventor, author, and Regents Professor of Chemical Engineering and Materials Science at the University of Minnesota. He is well known for his extensive work in surface science, detailed chemistry (microkinetics), chemical reaction engineering, catalysis, and renewable energy. He is also well known for mentoring over a hundred graduate students and his work on millisecond reactors and reactive flash volatilization.
Chemical reaction engineering is a specialty in chemical engineering or industrial chemistry dealing with chemical reactors. Frequently the term relates specifically to catalytic reaction systems where either a homogeneous or heterogeneous catalyst is present in the reactor. Sometimes a reactor per se is not present by itself, but rather is integrated into a process, for example in reactive separations vessels, retorts, certain fuel cells, and photocatalytic surfaces. The issue of solvent effects on reaction kinetics is also considered as an integral part.

Arvind Varma was the R. Games Slayter Distinguished Professor, School of Chemical Engineering at Purdue University. His research interests are in chemical and catalytic reaction engineering, and new energy sources.
This bibliography of Rutherford Aris contains a comprehensive listing of the scientific publications of Aris, including books, journal articles, and contributions to other published material.

Yurii Shaevich Matros was a Soviet and American scientist in the field of chemical engineering, known for his achievement in the theory and practice of heterogeneous catalytic processes. He is acknowledged as a “Godfather” of realization of catalytic processes in forced unsteady state conditions. Matros developed a catalytic reactor with periodic changes of direction of flow rate in packed bed of catalyst. This reactor is widely known in scientific and applied literature as an example of an application of developed theory of forced unsteady processes. Yurii Matros possessed a full doctoral degree of science and was a professor.

Sivanandi Rajadurai, otherwise known as Mylaudy Dr. S. Rajadurai is a scientist in the field of catalysis, physical chemistry, and emission control, focused on protection of the global environment and is a corporate executive. He is the chairman and founder of the Rajadurai Foundation.
Gilbert F. Froment is a Belgian Professor Emeritus of chemical engineering at Ghent University, Belgium, and a research professor at Texas A&M University. His career specialized in the fields of kinetic and chemical reaction engineering studies, including its application in process industry.
Chemical looping reforming (CLR) and gasification (CLG) are the operations that involve the use of gaseous carbonaceous feedstock and solid carbonaceous feedstock, respectively, in their conversion to syngas in the chemical looping scheme. The typical gaseous carbonaceous feedstocks used are natural gas and reducing tail gas, while the typical solid carbonaceous feedstocks used are coal and biomass. The feedstocks are partially oxidized to generate syngas using metal oxide oxygen carriers as the oxidant. The reduced metal oxide is then oxidized in the regeneration step using air. The syngas is an important intermediate for generation of such diverse products as electricity, chemicals, hydrogen, and liquid fuels.
Alexis Tarassov Bell is an American chemical engineer. He is currently the Dow professor of Sustainable Chemistry in the Department of Chemical and Biomolecular Engineering in UC Berkeley's college of chemistry. He is also the Faculty Senior Scientist at Lawrence Berkeley National Laboratory. He is known for his work with heterogenous catalysts and characterizing the mechanisms of these reactions on a quantum level.
References
- ↑ "University of Houston Honors Professor Dan Luss". Archived from the original on December 22, 2017. Retrieved December 28, 2012.
- ↑ "National Academy of Engineering" . Retrieved December 28, 2012.
- ↑ "AIChE Honors Professor Dan Luss" . Retrieved December 28, 2012.
- ↑ "ISCRE History of Amundson Award Winners". Archived from the original on December 10, 2015. Retrieved December 28, 2012.
- ↑ "Dan Luss Faculty Page at the University of Houston" . Retrieved December 28, 2012.
- ↑ "Professor Luss at the U. Houston Dept. of Chemical and Biomolecular Engineering" . Retrieved December 28, 2012.
- ↑ Luss, Dan; Amundson, Neal R. (1967). "Uniqueness of the steady state solutions for chemical reactor occurring in a catalyst particle or in a tubular reaction with axial diffusion". Chemical Engineering Science. 22 (3): 253–266. doi:10.1016/0009-2509(67)80113-1.
- ↑ Corbett, William E.; Luss, Dan (1974). "The influence of non-uniform catalytic activity on the performance of a single spherical pellet". Chemical Engineering Science. 29 (6): 1473–1483. doi:10.1016/0009-2509(74)80172-7.
- ↑ Pikios, Constantine A.; Luss, Dan (1977). "Isothermal concentration oscillations on catalytic surfaces". Chemical Engineering Science. 32 (2): 191–194. doi:10.1016/0009-2509(77)80104-8.
- ↑ Balakotaiah, Vemuri; Luss, Dan (1984). "Global analysis of the multiplicity features of multi-reaction lumped-parameter systems". Chemical Engineering Science. 39 (5): 865–881. doi:10.1016/0009-2509(84)85056-3.
- ↑ Martirosyan, Karen S.; Luss, Dan (2005). "Carbon combustion synthesis of complex oxides: Process demonstration and features". AIChE Journal. 51 (10): 2801–2810. doi: 10.1002/aic.10528 .
- ↑ Martirosyan, Karen S.; Chen, K.; Luss, Dan (2010). "Behavior features of soot combustion in diesel particulate filter". Chemical Engineering Science. 65: 42–46. doi:10.1016/j.ces.2009.01.058.
- ↑ Lane, Samuel L.; Luss, Dan (1993). "Rotating temperature pulse during hydrogen oxidation on a nickel ring". Physical Review Letters. 70 (6): 830–832. doi:10.1103/PhysRevLett.70.830. PMID 10054214.
- ↑ "Ceramic membrane reactor for synthesis gas production". ProQuest . Retrieved November 28, 2015.
- ↑ Zheng, Yang; Liu, Yi; Harold, Michael P.; Luss, Dan (2014). "LNT–SCR dual-layer catalysts optimized for lean NOx reduction by H2 and CO". Applied Catalysis B: Environmental. 148: 311–321. doi:10.1016/j.apcatb.2013.11.007.
- ↑ Nguyen, Hoang; Harold, Michael P.; Luss, Dan (2015). "Spatiotemporal behavior of Pt/Rh/CeO2/BaO catalyst during lean–rich cycling". Chemical Engineering Journal. 262: 464–477. doi:10.1016/j.cej.2014.09.103.