Related Research Articles

An oncogene is a gene that has the potential to cause cancer. In tumor cells, these genes are often mutated, or expressed at high levels.
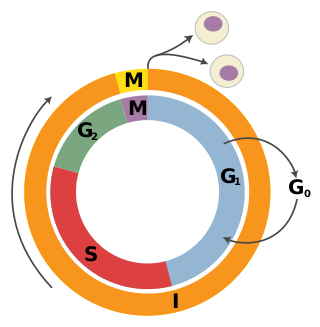
A tumor suppressor gene (TSG), or anti-oncogene, is a gene that regulates a cell during cell division and replication. If the cell grows uncontrollably, it will result in cancer. When a tumor suppressor gene is mutated, it results in a loss or reduction in its function. In combination with other genetic mutations, this could allow the cell to grow abnormally. The loss of function for these genes may be even more significant in the development of human cancers, compared to the activation of oncogenes.

Metastasis is a pathogenic agent's spread from an initial or primary site to a different or secondary site within the host's body; the term is typically used when referring to metastasis by a cancerous tumor. The newly pathological sites, then, are metastases (mets). It is generally distinguished from cancer invasion, which is the direct extension and penetration by cancer cells into neighboring tissues.

Loss of heterozygosity (LOH) is a type of genetic abnormality in diploid organisms in which one copy of an entire gene and its surrounding chromosomal region are lost. Since diploid cells have two copies of their genes, one from each parent, a single copy of the lost gene still remains when this happens, but any heterozygosity is no longer present.
Carcinogenesis, also called oncogenesis or tumorigenesis, is the formation of a cancer, whereby normal cells are transformed into cancer cells. The process is characterized by changes at the cellular, genetic, and epigenetic levels and abnormal cell division. Cell division is a physiological process that occurs in almost all tissues and under a variety of circumstances. Normally, the balance between proliferation and programmed cell death, in the form of apoptosis, is maintained to ensure the integrity of tissues and organs. According to the prevailing accepted theory of carcinogenesis, the somatic mutation theory, mutations in DNA and epimutations that lead to cancer disrupt these orderly processes by interfering with the programming regulating the processes, upsetting the normal balance between proliferation and cell death. This results in uncontrolled cell division and the evolution of those cells by natural selection in the body. Only certain mutations lead to cancer whereas the majority of mutations do not.

Kisspeptins are proteins encoded by the KISS1 gene in humans. Kisspeptins are ligands of the G-protein coupled receptor, GPR54. Kiss1 was originally identified as a human metastasis suppressor gene that has the ability to suppress melanoma and breast cancer metastasis. Kisspeptin-GPR54 signaling has an important role in initiating secretion of gonadotropin-releasing hormone (GnRH) at puberty, the extent of which is an area of ongoing research. Gonadotropin-releasing hormone is released from the hypothalamus to act on the anterior pituitary triggering the release of luteinizing hormone (LH), and follicle stimulating hormone (FSH). These gonadotropic hormones lead to sexual maturation and gametogenesis. Disrupting GPR54 signaling can cause hypogonadotrophic hypogonadism in rodents and humans. The Kiss1 gene is located on chromosome 1. It is transcribed in the brain, adrenal gland, and pancreas.

Maspin is a protein that in humans is encoded by the SERPINB5 gene. This protein belongs to the serpin superfamily. SERPINB5 was originally reported to function as a tumor suppressor gene in epithelial cells, suppressing the ability of cancer cells to invade and metastasize to other tissues. Furthermore, and consistent with an important biological function, Maspin knockout mice were reported to be non-viable, dying in early embryogenesis. However, a subsequent study using viral transduction as a method of gene transfer was not able to reproduce the original findings and found no role for maspin in tumour biology. Furthermore, the latter study demonstrated that maspin knockout mice are viable and display no obvious phenotype. These data are consistent with the observation that maspin is not expressed in early embryogenesis. The precise molecular function of maspin is thus currently unknown.

The KiSS1-derived peptide receptor is a G protein-coupled receptor which binds the peptide hormone kisspeptin (metastin). Kisspeptin is encoded by the metastasis suppressor gene KISS1, which is expressed in a variety of endocrine and gonadal tissues. Activation of the kisspeptin receptor is linked to the phospholipase C and inositol trisphosphate second messenger cascades inside the cell.
Protein S100-A4 (S100A4) is a protein that in humans is encoded by the S100A4 gene.

CD82, or KAI1, is a human protein encoded by the CD82 gene.

Protein NDRG1 is a protein that in humans is encoded by the NDRG1 gene.

Forkhead box protein O4 is a protein that in humans is encoded by the FOXO4 gene.

Reversion-inducing-cysteine-rich protein with kazal motifs, also known as RECK, is a human gene, thought to be a metastasis suppressor.

Breast cancer metastasis suppressor 1 is a protein that in humans is encoded by the BRMS1 gene.
A metastasis suppressor is a protein that acts to slow or prevent metastases from spreading in the body of an organism with cancer. Metastasis is one of the most lethal cancer processes. This process is responsible for about ninety percent of human cancer deaths. Proteins that act to slow or prevent metastases are different from those that act to suppress tumor growth. Genes for about a dozen such proteins are known in humans and other animals.

Transcription factor 21 (TCF21), also known as pod-1, capsuling, or epicardin, is a protein that in humans is encoded by the TCF21 gene on chromosome 6. It is ubiquitously expressed in many tissues and cell types and highly significantly expressed in lung and placenta. TCF21 is crucial for the development of a number of cell types during embryogenesis of the heart, lung, kidney, and spleen. TCF21 is also deregulated in several types of cancers, and thus known to function as a tumor suppressor. The TCF21 gene also contains one of 27 SNPs associated with increased risk of coronary artery disease.

NUAK family SNF1-like kinase 1 also known as AMPK-related protein kinase 5 (ARK5) is an enzyme that in humans is encoded by the NUAK1 gene.

Myosin-XVIIIb is a protein that in humans is encoded by the MYO18B gene.
Chromosomal instability (CIN) is a type of genomic instability in which chromosomes are unstable, such that either whole chromosomes or parts of chromosomes are duplicated or deleted. More specifically, CIN refers to the increase in rate of addition or loss of entire chromosomes or sections of them. The unequal distribution of DNA to daughter cells upon mitosis results in a failure to maintain euploidy leading to aneuploidy. In other words, the daughter cells do not have the same number of chromosomes as the cell they originated from. Chromosomal instability is the most common form of genetic instability and cause of aneuploidy.

J. William Harbour is an American ophthalmologist, ocular oncologist and cancer researcher. He is Chair of the Department of Ophthalmology at the University of Texas Southwestern Medical Center in Dallas. He previously served as the vice chair and director of ocular oncology at the Bascom Palmer Eye Institute and associate director for basic science at the Sylvester Comprehensive Cancer Center of the University of Miami's Miller School of Medicine.
References
- ↑ "Danny Welch and KUMC's new Department of Cancer Biology confront the complexities of metastasis". www.kumc.edu. Archived from the original on 2012-01-03.
- ↑ Goodman, Troy (Spring 2010). "Great Debates: Metastasis Mystery". UAB Magazine. University of Alabama at Birmingham.
- ↑ "Danny Welch: The "M" Word". Cancer History Project. Retrieved 2023-10-23.
- ↑ Comninos, Alexander N.; Dhillo, Waljit S. (Summer 2018). "Kisspeptin: the master regulator of reproduction?". The Endocrinologist (128).
- ↑ Pasquier J, Kamech N, Lafont AG, Vaudry H, Rousseau K, Dufour S (June 2014). "Molecular evolution of GPCRs: Kisspeptin/kisspeptin receptors". Journal of Molecular Endocrinology. 52 (3): T101-17. doi: 10.1530/JME-13-0224 . PMID 24577719.
- ↑ "Cancer and Metastasis Reviews". Springer. Retrieved 2023-10-23.