Chemotaxis is the movement of an organism or entity in response to a chemical stimulus. Somatic cells, bacteria, and other single-cell or multicellular organisms direct their movements according to certain chemicals in their environment. This is important for bacteria to find food by swimming toward the highest concentration of food molecules, or to flee from poisons. In multicellular organisms, chemotaxis is critical to early development and development as well as in normal function and health. In addition, it has been recognized that mechanisms that allow chemotaxis in animals can be subverted during cancer metastasis. The aberrant chemotaxis of leukocytes and lymphocytes also contribute to inflammatory diseases such as atherosclerosis, asthma, and arthritis. Sub-cellular components, such as the polarity patch generated by mating yeast, may also display chemotactic behavior.

Sex is the trait that determines whether a sexually reproducing organism produces male or female gametes. Male plants and animals produce small mobile gametes, while females produce larger, non-motile ones. Organisms that produce both types of gametes are called hermaphrodites. During sexual reproduction, male and female gametes fuse to form zygotes, which develop into offspring that inherit traits from each parent.

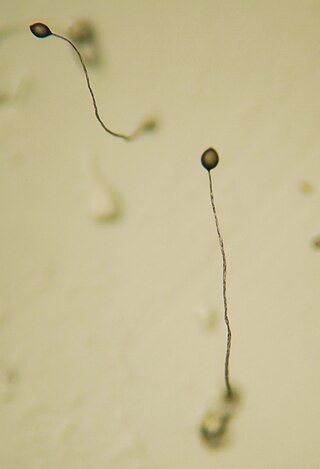
A flagellum is a hairlike appendage that protrudes from certain plant and animal sperm cells, and from a wide range of microorganisms to provide motility. Many protists with flagella are termed as flagellates.

Caenorhabditis elegans is a free-living transparent nematode about 1 mm in length that lives in temperate soil environments. It is the type species of its genus. The name is a blend of the Greek caeno- (recent), rhabditis (rod-like) and Latin elegans (elegant). In 1900, Maupas initially named it Rhabditides elegans. Osche placed it in the subgenus Caenorhabditis in 1952, and in 1955, Dougherty raised Caenorhabditis to the status of genus.
A taxis is the movement of an organism in response to a stimulus such as light or the presence of food. Taxes are innate behavioural responses. A taxis differs from a tropism in that in the case of taxis, the organism has motility and demonstrates guided movement towards or away from the stimulus source. It is sometimes distinguished from a kinesis, a non-directional change in activity in response to a stimulus.
Thermotaxis is a behavior in which an organism directs its locomotion up or down a gradient of temperature.
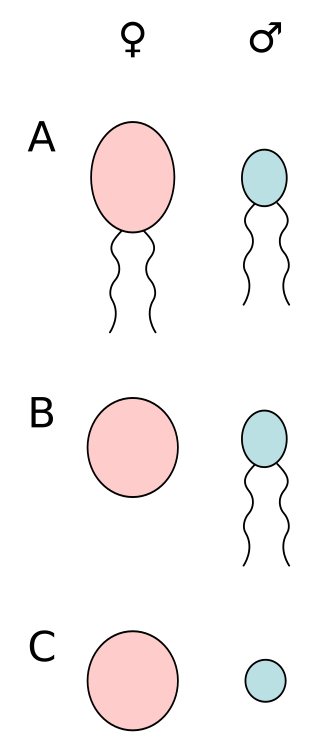
Anisogamy is a form of sexual reproduction that involves the union or fusion of two gametes that differ in size and/or form. The smaller gamete is male, a sperm cell, whereas the larger gamete is female, typically an egg cell. Anisogamy is predominant among multicellular organisms. In both plants and animals gamete size difference is the fundamental difference between females and males.

Oogamy is a form of anisogamy where the gametes differ in both size and form. In oogamy the large female gamete is immobile, while the small male gamete is mobile. Oogamy is a common form of anisogamy, with almost all animals and land plants being oogamous.
Julius Adler Ph.D. is an American biochemist. He has been an Emeritus Professor of biochemistry and genetics at the University of Wisconsin–Madison since 1997.

Major sperm protein (MSP) is a nematode specific small protein of 126 amino acids with a molecular weight of 14 kDa. It is the key player in the motility machinery of nematodes that propels the crawling movement/motility of nematode sperm. It is the most abundant protein present in nematode sperm, comprising 15% of the total protein and more than 40% of the soluble protein. MSP is exclusively synthesized in spermatocytes of the nematodes. The MSP has two main functions in the reproduction of the helminthes: i) as cytosolic component it is responsible for the crawling movement of the mature sperm, and ii) once released, it acts as hormone on the female germ cells, where it triggers oocyte maturation and stimulates the oviduct wall to contract to bring the oocytes into position for fertilization. MSP has first been identified in Caenorhabditis elegans.
Dictyostelium discoideum is a species of soil-dwelling amoeba belonging to the phylum Amoebozoa, infraphylum Mycetozoa. Commonly referred to as slime mold, D. discoideum is a eukaryote that transitions from a collection of unicellular amoebae into a multicellular slug and then into a fruiting body within its lifetime. Its unique asexual lifecycle consists of four stages: vegetative, aggregation, migration, and culmination. The lifecycle of D. discoideum is relatively short, which allows for timely viewing of all stages. The cells involved in the lifecycle undergo movement, chemical signaling, and development, which are applicable to human cancer research. The simplicity of its lifecycle makes D. discoideum a valuable model organism to study genetic, cellular, and biochemical processes in other organisms.

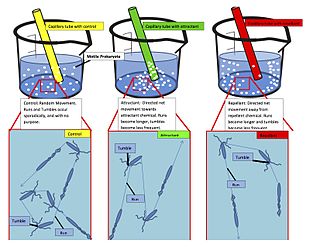
Bacterial motility is the ability of bacteria to move independently using metabolic energy. Most motility mechanisms which evolved among bacteria also evolved in parallel among the archaea. Most rod-shaped bacteria can move using their own power, which allows colonization of new environments and discovery of new resources for survival. Bacterial movement depends not only on the characteristics of the medium, but also on the use of different appendages to propel. Swarming and swimming movements are both powered by rotating flagella. Whereas swarming is a multicellular 2D movement over a surface and requires the presence of surfactants, swimming is movement of individual cells in liquid environments.

Phototaxis is a kind of taxis, or locomotory movement, that occurs when a whole organism moves towards or away from a stimulus of light. This is advantageous for phototrophic organisms as they can orient themselves most efficiently to receive light for photosynthesis. Phototaxis is called positive if the movement is in the direction of increasing light intensity and negative if the direction is opposite.
Sperm guidance is the process by which sperm cells (spermatozoa) are directed to the oocyte (egg) for the aim of fertilization. In the case of marine invertebrates the guidance is done by chemotaxis. In the case of mammals, it appears to be done by chemotaxis, thermotaxis and rheotaxis.
Sperm chemotaxis is a form of sperm guidance, in which sperm cells (spermatozoa) follow a concentration gradient of a chemoattractant secreted from the oocyte and thereby reach the oocyte.
Fungi are capable of a variety of behaviors. Nearly all secrete chemicals, and some of these chemicals act as pheromones to communicate with other individuals. Many of the most dramatic examples involve mechanisms to get fungal spores dispersed to new environments. In mushrooms, spores are propelled into the air space between the gills, where they are free to fall down and can then be carried by air currents. Other fungi shoot spores aimed at openings in their surroundings, sometimes reaching distances over a meter.
Aerotaxis is the movement caused by oxygen gradients. Positive aerotaxis involves the movement toward higher concentration of environmental oxygen, while negative aerotaxis involves the movement towards a lower concentration of environmental oxygen. Aerotactic bacteria gather around sources of air forming aerotactic bands.

Caenorhabditis elegans- microbe interactions are defined as any interaction that encompasses the association with microbes that temporarily or permanently live in or on the nematode C. elegans. The microbes can engage in a commensal, mutualistic or pathogenic interaction with the host. These include bacterial, viral, unicellular eukaryotic, and fungal interactions. In nature C. elegans harbours a diverse set of microbes. In contrast, C. elegans strains that are cultivated in laboratories for research purposes have lost the natural associated microbial communities and are commonly maintained on a single bacterial strain, Escherichia coli OP50. However, E. coli OP50 does not allow for reverse genetic screens because RNAi libraries have only been generated in strain HT115. This limits the ability to study bacterial effects on host phenotypes. The host microbe interactions of C. elegans are closely studied because of their orthologs in humans. Therefore, the better we understand the host interactions of C. elegans the better we can understand the host interactions within the human body.
Michael Eisenbach, Ph.D., is an Israeli biochemist who specializes in the navigation mechanisms of bacterial and sperm cells. He is a professor emeritus at the Weizmann Institute of Science, Department of Biomolecular Sciences, Rehovot, Israel. He discovered that sperm cells (spermatozoa) of mammals are actively guided to the egg. This opened the research field of mammalian sperm navigation. He demonstrated that the active navigation entails chemotaxis and thermotaxis. He made seminal contributions to the understanding of these two processes at the molecular, physiological and behavioural levels, as well as contributing to our understanding of the molecular mechanism of bacterial chemotaxis.

Run-and-tumble motion is a movement pattern exhibited by certain bacteria and other microscopic agents. It consists of an alternating sequence of "runs" and "tumbles": during a run, the agent propels itself in a fixed direction, and during a tumble, it remains stationary while it reorients itself in preparation for the next run.










